General Laboratory Safety
Faculty of Engineering and Natural Sciences ensures a safe work environment for its students, faculty members, staff and visitors. The primary goal of the Faculty is to minimize the risks of injury and illness to people work in the laboratory by providing sufficient training, tools and equipments.
With a specific end goal to evaluate the requirement for PPE the accompanying steps ought to be taken:
Survey
In the area being referred to identify sources of dangers, conduct a walk-through survey.
Categories for consideration:
- Impact
- Penetration
- Compression (roll-over)
- Chemical
- Heat
- Harmful dust
- Light (optical) radiation
- Drowning
- Falling
Sources
During the walk-through survey LSS, the Responsible Faculty Member and the university health and safety specialist should observe:
- Sources of motion; for instance, machinery or procedures where any development of instruments, machine components or particles could exist, or development of work force that could bring about impact with stationary articles
- Sources of high temperatures that could result in burns, eye injury or ignition of protective equipment
- Types of chemical exposures
- Sources of harmful dust
- Sources of light radiation, for instance, welding, brazing, cutting, heat treating, furnaces, and high intensity lights
- Sources of falling objects or potential for dropping objects
- Sources of sharp objects which might pierce or cut the hands
- Sources of rolling or pinching objects which could crush the feet
- Layout of work place and location of co-workers
- Any electrical hazards
- Review injury/accident data to help identify problem areas
Arrange information. Completing the walk-through survey, it is important to sort out the information and other data acquired. That material gives the premise to peril evaluation that empowers the lab user to choose the proper PPE.
Examine information. Having assembled and composed information with respect to a specific occupation, lab specialist need to assess the potential for wounds. Each of the distinguished hazards ought to be looked into and delegated to its sort, the level of danger, and the reality of any potential injury. Where it is predictable that a representative could be presented to a few perils at the same time, the outcomes of such exposure should be considered.
On the table below (Table 3.2), eye and face protection equipments can be seen.
Table 3.2 Eye and Face Protection | |||
|---|---|---|---|
| Safety Glasses | Splash goggles | Laser goggles | Face shields |
| Safety glasses provide eye protection from moderate impact and particles associated with grinding, sawing, scaling, broken glass, and minor chemical splashes, etc. | Splash goggles provide adequate | The lens of the eyewear is a | Face shields provide additional protection |
 |  |  |  |
(Images courtesy of Egebant)
Adopted from Cornell University Environmental Health & Safety Department.
Eye Protection
The use of contact lenses is not recommended while working with chemicals that cause eye irritation.
Eye protection should be worn at all times while working with hazardous chemicals/biological materials or any physical hazards in the laboratory. Visitors should be provided with temporary protective goggles or, at least, protective glasses if they are allowed in any area in which the occupational use of eye protection is required.
Using contact lenses:
In the event of a chemical accident to the eyes, there could be some protection but, on the other hand, the presence of the lens would be an impediment to prompt and thorough flushing of the eyes. The lens would have to be removed which might result in damage to the eye in itself. If, however, the wearer of contact lens conscientiously wears a good-quality pair of goggles at all times when there is a possibility of an incident occurring, there is probably little risk in wearing contact lens. Even in the latter case, where extremely corrosive vapors are likely to be involved, there is a possibility of capillary action causing these vapors to be drawn under the contact lens, and the wearer should exercise caution if there is any suspicion that this could happen.
Safety glasses
Safety glasses provide eye protection from moderate effect and particles connected with grinding, sawing, scaling, broken glass, and minor chemical splashes, and so forth. Side defenders are required when there is a risk from flying items. In prescription form for those people requiring corrective lenses, safety glasses are accessible. In the case of safety glasses don't give sufficient insurance to procedures that include substantial synthetic utilize, such as, blending, pouring, or blending, splash goggles should be utilized.
Splash goggles
Including potential chemical splash hazards, utilization of concentrated corrosive material, and bulk chemical transfer splash goggles give satisfactory eye protection from numerous dangers. Goggles are available with clear or tinted lenses, fog proofing, and vented or non-vented frames. Be aware that goggles intended for carpentry (can be recognized by the various small holes throughout the face piece) are not fitting for working with chemicals. In the event of a splash, chemicals could enter into these small holes, and result in a chemical exposure to the face. Ensure the goggles you pick are evaluated for use with chemicals.
Laser goggles
The lens of the eyewear is a filter/absorber designed to reduce light transmittance of a specific wavelength. The lens can filter out (or absorb) a specific wavelength while maintaining adequate light transmission for other wavelengths.
A single pair of safety glasses is not available for protection from all LASER outputs. The type of eye protection required is dependent on the spectral frequency or specific wavelength of the laser source. See the Laser Safety section for more information.
Face Protection
Face shields
When utilized in combination with safety glasses or splash goggles, face shields provide additional protection to the eyes and face. Face shields comprise of a flexible headgear and face shield of tinted or clear lenses or a mesh wire screen. When the whole face needs assurance, they ought to be utilized as a part of operations and worn to shield the eyes and face from flying particles, metal sparks, and chemical/biological splashes. Face shields with a mesh wire screen are not appropriate for use with chemicals. Face shields must not be used alone and are not a substitute for appropriate eyewear and they should always be worn in conjunction with an essential type of eye protection, for example, safety glasses or splash goggles.
Accidents that cause head injuries are difficult to anticipate or control. If hazards exist that could cause head injury, employees should try to eliminate the hazards, but they should also wear head protection.
Safety hats protect the head from impact, penetration, and electrical shock. Head protection is necessary if you work where there is a risk of injury from moving, falling, or flying objects or if you work near high-voltage equipment.

Figure 3.1 Head cover
(Courtesy of Egebant)
Hard hats (Figure 3.1) should be water resistant, flame resistant, and adjustable. Follow these guidelines for head safety:
- Check the shell and suspension of your headwear for damage before each use.
- Look for cracks, dents, gouges, chalky appearance, and torn or broken suspension threads.
- Discard damaged hats or replace broken parts with replacements from the original manufacturer.
Most accidents involving hands and arms can be classified under four main hazard categories:
- Chemicals
- Abrasions
- Cuts
- Heat/cold
There are several types of gloves that provide protection against and opposes corruption and pervasion to chemicals. Confiding in the type and concentration of the chemical, performance characteristics of the gloves, conditions and duration of use, hazards present, and the duration of time a chemical has been in contact with the glove, all gloves must be replaced periodically.
Gloves must be worn at any potential danger like chemicals, cuts, lacerations, abrasions, punctures, burns (heat/cold), biological materials, or harmful temperature extremes and when utilizing chemicals that are easily ingested through the skin and/or particularly hazardous. The correct utilization of hand protection can shield from potential chemical and physical hazards
Selecting the Proper Gloves
Proper selection of the glove material is essential to the performance of the glove as a barrier to chemicals/biological materials/physical hazards. Several properties of both the glove material and the chemical/biological material/physical hazard with which it is to be used should influence the choice of the glove. Some of these properties include: permeability of the glove material, breakthrough time of the chemical, temperature of the chemical, type of the possible physical hazard, thickness of the glove material, and the amount of the chemical that can be absorbed by the glove material (solubility effect). Glove materials vary widely in respect to these properties; for instance, neoprene is good for protection against most common oils, aliphatic hydrocarbons, and certain other solvents, but is unsatisfactory for use against aromatic hydrocarbons, halogenated hydrocarbons, ketones, and many other solvents.
Please see Chemwatch for selecting the proper gloves.
Double Gloving
“Double-gloving” is a common practice used with disposable gloves. Twofold layer of assurance is provided by wearing two pairs of gloves on each other. If the outer glove becomes contaminated, starts to degrade, or tears open, until removing and replacing it, the inner glove continues to offer protection. The best practice is to check outer gloves frequently, watching for signs of degradation (change of color, change of texture, tears, etc.). At the first sign of degradation or contamination, always remove and dispose of the contaminated ones immediately and double-glove with a new set. If the inner glove appears to have any contamination or degradation, remove both pairs of gloves, and double glove with a new pair.
It is desirable to double glove with two sets of gloves made from different materials when working with mixtures of chemicals. If one chemical infuses through the outer glove material, the inner gloves can still protect by this method. The type of the glove materials should be chosen depending on the chemical worked with.
Glove Removal Precautions
Removing disposable gloves depends on simple rules: Firstly, grab the cuff of the left glove with the gloved right hand and remove the left glove. After that, while holding the removed left glove with the gloved right hand, insert a finger under the cuff of the right glove and gently invert the right glove over the glove in the palm of your hand and dispose of them properly. Finally, wash your hands with soap and water (See Figure 3.2).
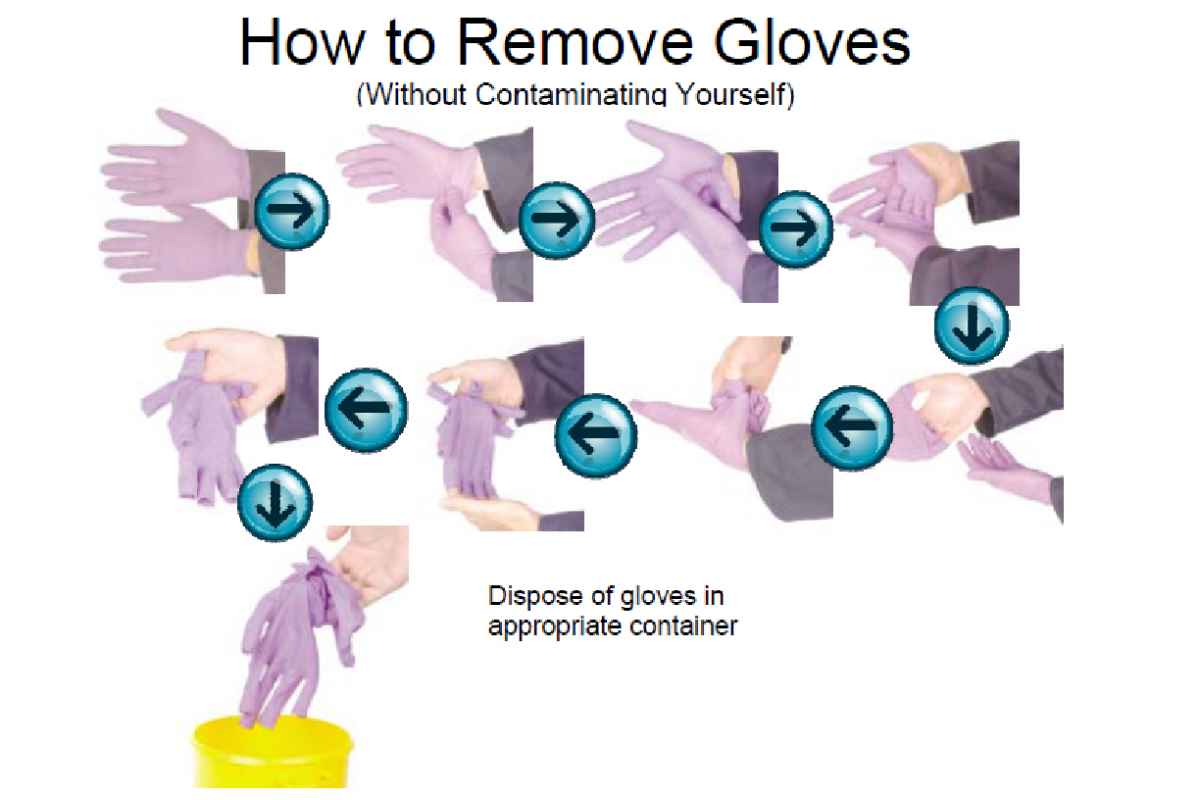
Types of Gloves
Table 3.3 represents the types of gloves:
| Table 3.3 Types of Gloves | ||
|---|---|---|
| Latex gloves | Resistant to ketones, alcohols, |  |
| Nitrile gloves | Resistant to alcohols, caustics, |  |
| Cryogenic gloves | Cryogenic gloves are used to protect |  |
| PVA Gloves | Resistant to chlorinated solvents, |  |
| Cut-resistant gloves | Cut resistant gloves are gloves |  |
| Heat-resistant gloves | Working with metal and glass forming |  |
Latex gloves
Natural Rubber Latex - Resistant to ketones, alcohols, caustics, and organic acids.
Due to the fact that latex gloves can degrade severely in seconds while in use with common chemicals, the use of them are not really encouraged. Latex contain several proteins, so latex gloves can also result in allergic reaction in some users. Symptoms can include nasal, eye, or sinus irritation, hives, shortness of breath, coughing, wheezing, or unexplained shock. Using latex gloves should be stopped if any of these symptoms become apparent.
The use of latex gloves is only appropriate for:
- Most biological materials.
- Nonhazardous chemicals.
- Clean room requirements.
- Medical or veterinary applications.
Very dilute, aqueous solutions containing < 1% for most hazardous chemicals or < 0.1% of a known or suspected human carcinogen.
Nitrile gloves
Nitrile - Resistant to alcohols, caustics, organic acids, and some ketones.
Cryogenic gloves
Cryogenic gloves are used to protect hands from extremely cold temperatures. These gloves should be used when handling dry ice and when dispensing or working with liquid nitrogen and other cryogenic liquids. For further information please consult Cryogenic Safety section.
PVA gloves
Polyvinyl alcohol (PVA) - Resistant to chlorinated solvents, petroleum solvents, and aromatics.
Cut-resistant gloves
Cut-resistant gloves are gloves designed to protect the wearer's hands from cuts while working with sharp tools.
Heat resistant gloves
Thermal safety is also another part of personal protective equipment. Working with metal and glass forming and hot surfaces requires gloves that offer the highest level of protection against the multiple hazards of a high-heat workplace.
Loose or torn clothing should be avoided without wearing a lab coat because of the ignition, absorption, and entanglement in machinery risks. Dangling jewellery, finger rings or other tight jewellery and excessively long hair should also be avoided.
Lab Coats
When properly used, lab coats (Figure 3.3.a):
- Provide protection of skin and personal clothing from incidental contact and small splashes.
- Prevent the spread of contamination outside the lab (provided they are not worn outside the lab).
- Provide a removable barrier in the event of an incident involving a spill or splash of hazardous substances.
There are no design or test criteria specified in regulations or guidelines specific to lab coats. What this means is that:
- Lab coats are not tested for normal conditions that may be experienced in a research lab with respect to chemical use, or joined research activities.
- Manufacturers of the lab coats do not provide information about the capability of a lab coat for a combination of hazards. If a coat is “flame resistant”, it may not be chemical resistant or acid resistant.
- If a coat is sold as flame resistant, this means it is not tested involving flammable chemicals on the coat. The flame resistance test criteria includes simulation of the possibilities of a flash fire, or electric arc flash, not a chemical fire. “Flame resistant” term refers to the characteristic of a fabric that avoids burning in air.


Figure 3.3 Lab coat (a) and apron (b) (Courtesy of Egebant)
Lab coats should be provided for protection and convenience. They should be worn at all times in the lab areas. Due to the possible absorption and accumulation of chemicals in the material, lab coats should not be worn in the lunchroom or elsewhere outside the laboratory.
Choosing the right lab coat
Lab coats are made of different materials, and depending on the type of hazard in the lab, it is significant to select the lab coat. Determination of the type of hazard in the lab is the first step in this selection process.
Some questions to consider are the following:
- Do you primarily work with chemicals, biological agents, radioisotopes, or a mix of things?
- Are there large quantities of flammable materials (>4 liters) used in a process or experiment?
- Are there water reactive or pyrophoric materials used in the open air, e.g. in a fume hood instead of a glove box?
- Are there open flames or hot processes along with a significant amount of flammables?
- How are hazardous chemicals used and what engineering controls are available, e.g. a fume hood or glove box?
- Is there a significant risk of spill, splash or splatter for the tasks being done?
- What is the toxicity of chemicals used and is there concern about careless spread of contamination?
One coat may not work for all lab operations. Users might need to provide a basic poly/cotton mixture coat for most operations, but have accessible lab coats of treated cotton or Nomex for work involving pyrophoric materials, extremely flammable chemicals, extensive amounts of flammable chemicals, or work around hot procedures or operations. If there is a possibility of a chemical splash, rubber apron over the flame resistant lab coat should also be used.
Flame resistant (FR) lab coats
Work with pyrophoric, spontaneously combustible, or extremely flammable chemicals presents an especially high potential for fire and burn risks to the skin. The use of fire retardant or fire resistant (FR) lab coats is recommended to provide additional skin protection where the individual will be working with these chemicals. The primary materials used for FR lab coats are FR-treated cotton or Nomex.
Lab coat use
When lab coats are in use, the following should be observed:
- Wear lab coats that fit properly. Lab coats are available in a variety of sizes. Some lab coat services also offer custom sizes (e.g., extra long sleeves, tall, or woman’s fit). Lab coats should fasten close to the collar to provide optimal protection.
- Lab coats should be worn fully buttoned or snapped with sleeves down.
- Wear lab coats only when in the lab or work area. Remove lab coats when leaving the lab/work area to go home, to lunch, to the restroom, or meetings in conference rooms, etc.
Laundry services are not equipped to handle significant contamination of lab coats with hazardous materials. In the event of a significant spill of a hazardous material on the lab coat, remove the coat immediately. If skin or personal clothing is impacted, it will be necessary to proceed to an emergency shower. Remove any contaminated clothing, and shower. Generally, significantly contaminated coats and clothing will be considered a hazardous waste, and must be managed based on the type of contamination.
Lab coat cleaning
Lab coats must not be cleaned at home. For lab coat cleaning, please contact LS.
Aprons
In the case of some procedures in the laboratory, such as washing glassware, large quantities of corrosive liquids in open containers are handled. In this situation, plastic or rubber aprons should be worn over the lab coat.
A high-necked, calf- or ankle-length, rubberized laboratory apron (See Figure 3.3.b) or a long-sleeved, calf- or ankle-length, chemical- and fire-resistant laboratory coat should be worn whenever laboratory manipulation or experimentation is conducted.
A respirator is a device designed to protect the wearer from inhalation of harmful substances.
When chosen correctly and used properly, respirators can protect the wearer from,
- Fumes and smokes (welding fume)
- Harmful dusts (lead, silica, and other heavy metals)
- Gases and vapors (chemical exposures)
- Oxygen deficiency (oxidation, displacement, and consumption)
- Biological hazards (tuberculosis, whooping cough, flu viruses)
Inspection
Users must inspect their respirators before and after use. Respirator inspections must include checking that;
- Sealing surface are clean and free of cracks and holes
- Rubber and elastic parts have good pliability and no signs of deterioration
- Inhalation and exhalation valves are clean and seated properly
- Straps are sufficiently elastic and free of worn areas
- If full face, face shield is cleaned and clear (no smudges, scratches, or other damage that may impede visibility)
Respirators that fail an inspection must be removed from service and replaced.
Before using a respirator, the wearer must perform a positive and negative pressure check. The wearer must ensure current facial condition will allow an effective seal (for example the wearer must be clean shaved).
Positive pressure check. Close off the exhalation valve with palms and exhale gently. No leakage outward around the seal should occur.
Negative pressure check. Close off the cartridges and inhale. The respirator should collapse slightly on the face. No leakage around the face seal should occur while maintaining a negative pressure inside the respirator for several seconds.
Maintenance
Cleaning
Respirators must be cleaned and disinfected after each use as follows:
- Remove filters or cartridges.
- Disassemble and wash with mild dishwashing detergent in warm water, using a soft brush.
- Thoroughly rinse to remove any detergent residue.
- When the cleaner used does not contain a disinfecting agent, respirator components must be immersed for two minutes in a sodium hypochlorite (30 mL household bleach in 7.5 L of water) solution, or other disinfectant. The solution used to clean the respirator(s) should contain some type of biocide for disinfection. Rinse in fresh, warm water.
Do not use organic solvents to clean a respirator or high heat to dry it, as this may damage the elastomeric face piece.
Cartridges and filters
- Change cartridges and filters according to the specific schedule provided with the authorization, or sooner if you experience an increased resistance in breathing or when you detect contaminant odors or taste while wearing your respirator.
- General guidance for organic vapor cartridges. Lab users who use respirators intermittently and perhaps in different environments should never reuse organic vapor cartridges after one use. This is due to chemical desorption of the vapors/gases and their migration through the cartridge charcoal bed. When this occurs, contaminants could be inhaled by the respirator wearer upon initial donning and the concentration could even be higher than contaminant concentrations found in the ambient workplace atmosphere.
Replacement and repairs
Repair of respirators may be done only by experienced personnel with parts designed for the specific respirator needing repair. No attempt may be made to replace parts or to make adjustments or repairs beyond the manufacturer’s recommendations.
Storage
- Store respirators away from dust, sunlight, heat, extreme cold, excessive moisture, damaging chemicals, or contamination.
- Filters and cartridges must be removed from the respirator and stored in separate bags to prevent cross-contamination.
- Do not store items on top of respirators, which could deform the shape of the face piece.
- Do not store respirators in such places as lockers or tool boxes unless they are on a separate shelf or in carrying cases or cartons to preserve the shape of the face piece.
- Respirators must be packed and stored according to the manufacturer’s instructions.
- Never store a respirator within a fume hood or at a workbench where contaminants are present.
Maintenance and Care of Dust Masks
Dust masks must be maintained in a clean and sanitary condition. Users who wear dust masks must
- Store dust masks in a plastic bag or box in a secure location such as a locker or desk drawer, away from moisture and contamination.
- Not share dust masks with others.
- Not use a dust mask that is torn, distorted, or dirty.
Types of Masks
| Table 3.4 Types of Masks | ||
|---|---|---|
| Dust mask | The use of the term “dust” mask for the non-rigid soft felt mask is somewhat of a misnomer since, in modified forms, they can be used for other applications such as limited protection against paint fumes, moderate levels of organics, acid fumes, mercury, etc., although their biggest use is against nuisance dust. |

|
| Half face respirator | The half-face cartridge respirator is the type most frequently used, especially in atmospheres in which there is little or no problem of irritation or absorption of material through the skin. |  |
| Full face respirator | Full-face air-purifying respirators are similar in many respects to half-face respirators, with the obvious difference that the mask covers the upper part of the face, protecting the eyes. |  |
Dust mask
The use of the term “dust” mask for the non-rigid soft felt mask is somewhat of a misnomer since, in modified forms, they can be used for other applications such as limited protection against paint fumes, moderate levels of organics, acid fumes, mercury, etc., although their biggest use is against nuisance dust.
These units are the simplest form of the air-purifying respirator. These respirators normally should not be employed for hazardous dusts, but are helpful for exposures to inert or nuisance dust levels below 15 mg/m3.
Half face respirator
The half-face cartridge respirator is the type most frequently used, especially in atmospheres in which there is little or no problem of irritation or absorption of material through the skin. The facepiece of most of these units is molded of a flexible plastic or silicone rubber, which provides a seal to the face when properly adjusted. As noted earlier, facial hair between the mask and the face will prevent the seal from being effective, and it is not permitted for a person with a beard or extended sideburns in the area of the seal to be fitted with a respirator. Accommodation for individuals who wear glasses also must not break the seal to the face. The face pieces of most brands of these units are provided with receptacles for two sets of cartridges and/or filters. The respirators are certified as complete units, i.e., the facepiece is equipped with specific filters. Cartridges from one vendor cannot be used on another manufacturer's facepiece. The major advantage of this type of unit is that by interchanging cartridges and filters, or by using one or more additional filters and cartridges in series, a single face piece can be adapted to provide protection against a large variety of contaminants.
Full face respirator
Full-face air-purifying respirators are similar in many respects to half-face respirators, with the obvious difference that the mask covers the upper part of the face, protecting the eyes.
In laboratories, laboratory support areas, and other areas where chemical, biological and physical hazards are present, foot protection should be supplied at all times. Wearing sandals or similar types of perforated or open-toed shoes when working with or around hazardous chemicals or physical hazards must be avoided.
This is due to the potential exposure to toxic chemicals and the potential associated with physical hazards such as dropping pieces of equipment or broken glass being present. In general, shoes should be comfortable, and leather shoes are preferable to cloth shoes due to the better chemical resistance of leather compared to cloth. Leather shoes also tend to absorb fewer chemicals than cloth shoes. However, leather shoes are not designed for long term exposure to direct contact with chemicals. In such instances, chemically resistant rubber boots are necessary.
In some cases, the use of steel-toed shoes (Figure 3.5) may be appropriate when heavy equipment or other items are involved. Chemically resistant boots or shoe covers may be required when working with large quantities of chemicals and the potential exists for large spills to occur.
Contact LS to obtain foot protection.


(Courtesy of Egebant)
References
- Furr, A. K. (2000). CRC Handbook of Laboratory Safety. (5th ed.). United States of America: CRC Press LLC.
- Georgia Institute of Technology Laboratory Safety Manual. (2013, April).
- Cornell University Laboratory Safety Manual and Chemical Hygiene Plan. (2006, January).
- Lab Coat Selection, Use, and Care at MIT. (2013, September). Retrieved from https://labcoats.mit.edu/guidance
- Harvard University PPE Selection Guide. (2015, April). Retrieved from https://www.ehs.harvard.edu/sites/ehs.harvard.edu/files/ppe_selection_gu...
- Stanford University Respiratory Protection Program. (2015, March).
- Texas A&M University Laboratory Safety Manual. (2009, February).
- University College London Personal Protective Equipment Policy & Guidance. (2015, March).
- United States Department of Labor, Occupational Safety & Health Administration, Standards. (2015, March).
Regarding possible noise hazards, there is a regulation about the Noise safety in Turkey. According to this regulation, the highest exposure value is LEX, 8h = 85 dB (A) and Ppeak = 140 µ Pa.
If you work in a high noise area, wear hearing protection. Most hearing protection devices have an assigned rating that indicates the amount of protection provided.
Depending on your level of exposure, you may choose from the following devices (Figure 3.4):
- Disposable earplugs
- Reusable earplugs
- Headband plugs
- Sealed earmuffs


Figure 3.4 Headband/ear muff and ear plug (Courtesy of Egebant)
Earplugs may be better in hot, humid, or confined work areas. They may also be better for lab users who wear other PPE, such as safety glasses or hats. Earmuffs, on the other hand, may be better for users who move in and out of noisy areas, because the muffs are easier to remove. Before resorting to hearing protection, attempt to control noise levels through engineering or operational changes.
To avoid contamination, follow these guidelines when using earplugs:
- Wash your hands before inserting earplugs.
- Replace disposable earplugs after each use.
- Clean reusable earplugs after each use.
Administrative controls include policies and procedures that help develop a safe laboratory work practices. It sets a standard of measures within a laboratory. These measures are taken to prevent accidents and hazards that do not involve engineering controls or PPE. All users working in the laboratories should adhere administrative regulations implemented by faculty. Along with LS, Responsible Faculty Member is in charge to make sure that lab users are aware of all possible hazards in a laboratory, including but not limited to chemical biological, physical, and electrical hazards. For further information, see the Safety Training section.
Faculty programs are in charge to develop regulations and guidelines to develop a safety system to protect lab users against several hazards arising from several sources such as hazardous chemicals and physical hazards.
Faculty programs are strongly encouraged to integrate the rules and regulations explained in this manual, besides meeting the regulatory requirements. This laboratory safety manual deals with minimum requirements to meet safe laboratory standard criteria. In addition to these regulations, faculty programs, Responsible Faculty Member and LS/LSS have the authority to implement more detailed policies.
Orientation And Training
All newly arrived employees/students are obliged to go through mandatory orientation conducted by laboratory specialist (see laboratory specific orientation form, Appendix 5.1. upon assessment of training needs of the individual conducted by laboratory safety specialist and having successfully completed the training. Newcomers are required to contact the laboratory safety specialist and undergo orientation upon arrival in order to acquire access to laboratory facilities and become familiar with university policy. For more information please refer to Safety Training section of this handbook.
The Governmental Safety Regulation requires that the general practice include specific elements and measures to ensure users’ protection in the laboratory. One of these measures is standard operating procedures (SOPs) "relevant to safety and health considerations to be followed when laboratory work involves the use of hazardous chemicals."
SOPs can be presented as stand-alone documents or as supplementary information to a research notebook or research proposals. SOPs ensure that a process is performed meeting required health and safety measures.
An SOP should involve the items listed below:
- The chemicals involved and their hazards.
- Special hazards and circumstances.
- Use of engineering controls (such as fume hoods).
- Required PPE.
- Spill response measures.
- Waste disposal procedures.
- Decontamination procedures.
- Description of how to perform the experiment or operation.
In addition to preparing SOPs for working with hazardous chemicals as a minimum requirement of laboratory standard, LSs are encouraged to develop additional SOPs regarding operation of each piece of equipment present in the laboratory that poses the hazards related to working with the equipment. Some examples to this include:
- Safe use and considerations of lasers.
- Use of cryogenic liquids and fill procedures.
- Use of equipment with high voltage.
An SOP does not need to be written as a long dissertation. It is advisable to refer other sources of information. Some examples of SOPs include:
"Please see page 16 in the operator’s manual that can be found in file cabinet”. “Please review the chemical and physical hazards by reading its SDS (see ChemWatch) before using this chemical. “ Appropriate personal protective equipment can be selected by reviewing lab safety handbook, personal protective equipment section.”
While developing SOPs, laboratories can ask LS and LSS to assist. It is the responsibility of each laboratory to prepare SOPs and to make sure that procedures presented in are adequate to protect lab users who use hazardous chemicals. Due to the complexity of research conducted in a laboratory, LS and LSS are not in charge to prepare SOPs, but help improve them.
Responsible Faculty Member and LS/LSS are responsible for making sure that prepared SOPs are in agreement with health and safety considerations and involve necessary information about hazardous chemicals. They are also responsible for making sure that PPE and engineering controls meet the criteria to prevent exposure. Moreover, Responsible Faculty Member and LS/LSS should ensure that lab users had the necessary training on SOPs prepared.
Safety Signs
All laboratories are equipped with a safety board on every entrance door indicating hazards within the laboratory, general rules and required personal protective equipment (Figure 5.1). Furthermore, specific hazard and emergency area locations are indicated by individual sign boards/labels within the laboratory. All users are obliged to follow the rules and precaution measures indicated on these signs.

Procedural controls are useful to establish best management practices in a laboratory. These practices are useful in maintaining health and safety measures. In addition, they help increase the productivity in the laboratory by increasing the efficient use of lab space and the reliability of experiments (since contamination risk is less). By raising the awareness of lab users about health and safety regulations, implementation of best management practices also result in a decrease in number of accidents, injuries and spills. In this way, the overall responsibility of LS/LSS and Responsible Faculty Member also decreases. In summary, following practices are key to impose a safe work behaviour and help spread a safety culture within the laboratory.
Housekeeping practices add up to general condition and appearance of a laboratory. These include:
- Lab users should keep all areas of the lab free of trash, unused chemical containers, clutter and extraneous equipment. Lab areas include benches, hoods, refrigerators, cabinets, chemical storage cabinets, sinks, trash cans, etc.
- When not in use, containers of chemicals should be kept closed.
- All chemicals spills should be cleaned up as soon as possible. Additional splashes on the equipment, cabinets, doors, and benches around should also be checked while cleaning up the spill. Please refer to Chemical Spill section for more information on cleaning up spills.
- Areas around emergency exits, emergency equipment and devices should always be kept tidy. This rule also applies for eyewash/emergency showers, electric power panels, fire extinguishers, and spill cleanup supplies.
- As required, there should be a minimum 1-meter of space between benches and equipment. Emergency exits should be kept clear of any obstacles such as bottles, boxes, equipment, electric cords, etc. Never store combustible materials in exits, corridors or stairways.
- Heavy and bulky chemicals should be stored close to floor. The sprinkles should not be covered. There must be at least 45 cm distance between sprinkle and any item in the lab.
- Chairs and countertops are not intended to be used as stairs. Always use a stepladder to reach overhead items.
In summary, good housekeeping has obvious health and safety benefits and can have a positive mental effect on laboratory users who work in a clean environment, which can lead to increased productivity.
It should also be noted that, whether positive or negative, the first impression of the general condition of a lab has the most significant impact on a lab inspection test carried out by an agency.
Responsible Faculty Member and LS/LSS are responsible for maintaining a clean and healthy working environment and good housekeeping practices in the labs under their supervision.
Some general guidelines that should always be followed include:
- Eating, drinking, chewing gum or applying cosmetics in a lab is not allowed.
- Food or drinks cannot be stored in lab refrigerators used to store chemicals.
- Pipetting by mouth is strictly forbidden. This may give rise to ingestion of chemicals or inhalation of their vapor. A suction bulb should be used for pipetting purposes.
- Hair should be tied all the time. Loose clothing is preferred and wearing jewellery is not allowed.
- Always wear a lab coat while working with chemicals.
- Do not wear shorts and sandals in the lab, particularly when someone is using corrosive chemicals. Exposure may result in skin corrosion and burns. Dropped pieces or broken glass may cause injuries.
- In case of a chemical contamination, lab coats, gloves and other PPE should immediately be removed. Further chemical exposure may propagate from this equipment.
- Once contaminated PPE was removed, affected area should vigorously be washed with water for at least 15 minutes.
- Before leaving the lab, lab coat, scrubs, gloves, and other PPE (gloves particularly) should be removed. PPE should not be worn outside lab area, particularly in the places where food and drink are served.
- Wash your hand after removing the gloves and before leaving the lab. Do not touch other items such as phone, turning door knobs or do not use elevator before washing your hands.
- Lab coats must not be cleaned at home. For lab coat cleaning, please contact LS.
Smoking is prohibited in all lab areas.
Good chemical hygiene practices include the use of personal protective equipment (PPE) and good personal hygiene habits. Although PPE can offer a barrier of protection against chemicals and biological materials, good personal hygiene habits are essential to prevent chemical exposure, even when using PPE.
Food ingestion and chemically contaminated drinks are sources of chemical exposure. Bringing food or beverages or storing in chemical refrigerators or cabinets them causes such contamination. Storing food or beverages in chemical cabinets/refrigerators facilitate chemical absorption by vapor. Thus, chemical exposure takes place upon consuming food or beverages stored with chemicals. Therefore, eating or drinking in the lab is strictly forbidden.
Along with food and beverages, cosmetic products also have potential to absorb chemical vapor and dust. Applying cosmetics in the lab, including hand lotions, also enables chemical absorption and is another source of skin exposure, particularly in the areas where hazardous chemicals are stored.
To prevent exposure to hazardous chemicals through ingestion, do not eat, drink, chew gum, or apply cosmetics in areas where hazardous chemicals are used. Wash your hands thoroughly after using any chemicals or other laboratory materials, even if you were wearing gloves, and especially before eating or drinking
It should be noted that, although several chemicals show acute effects of exposure, effect of certain chemicals might not be seen in a long term even though exposed repeatedly (chronic exposure). Chemical exposure both by food beverages and/or cosmetics may have short term and long term effects.
Working alone is prohibited for research purposes, particularly involving hazardous chemicals and experimental procedures. Otherwise, LS or Responsible Faculty Member should prepare guidelines and standard operating procedures (see Standart Operating Procedures section) highlighting working alone principles, notifying procedures and instances when working alone is strictly forbidden. In cases where working alone is a must, LS/LSS or Responsible Faculty Member should approve the work and experimental setup. Whole procedure should be monitored by surveillance system.
It is strongly advisable to notify someone in the working area, in the next room or in the same floor. A “buddy system” may be established where buddy person performs routine checks on alone users periodically. This is to make sure that a safe experimental procedure is followed. Routine checks may be carried out either by physically or via a phone. A buddy should not be in the same room when the person working alone is carrying out a highly hazardous work. Visual check systems is another option to make sure that working alone is being performed safely or to determine if help is needed.
If an emergency event takes place that involves highly hazardous chemicals and buddy had to leave the lab before the end of experimental procedure, the buddy should notify security (7555). Notification includes name of the person working alone, location of the accident and the time of accident. In addition, buddy warns the person conducting the experiment involving hazardous chemicals so that the person can finish the experiment in the safest manner possible and inform security personnel on the latest condition of the experiment. It is strictly forbidden to use security personnel as a buddy. This will impair the safety of all the people involved.
Please note: For rooms that are locked due to security needs, prior arrangements are required to allow the designated buddy access. It is also important to understand that if the door to the lab does not have a window, or if the window is covered, then there is a chance that if something happened to a person working alone in a locked lab, then they may not be discovered until someone else from the lab goes into the room (which could be a day or more later).
Instances when working alone is permitted includes:
- Office work
- Housekeeping activities
- Assembly or modification of laboratory apparatus. Here, no chemical, electrical, or other physical hazards should be present.
- Routine lab functions as a part of a standard operating procedure.
Instances when a “buddy system” is needed includes:
- Toxic or otherwise hazardous chemicals included experiments
- Experiments involving high-pressure equipment
- Experiments involving large quantities of cryogenic materials
- Experiments involving work with unstable (explosives) materials
- Experiments involving Class 3 or 4 Lasers
- Transfer of large quantities of flammable materials, acids, bases, and other hazardous materials
- Changing out compressed gas cylinders containing hazardous materials
Setting up procedures and policies for working alone is LS’s or Responsible Faculty Member’s responsibility. Every lab user working under the corresponding faculty member’s supervision should follow these procedures and policies.
It is strongly advised to establish a communication tool in the lab in order to inform authorities in an emergency situation. This can be done by various means including a wired phone or a cellular phone (if available), or by a two-way radio. It is advised to post a sign highlighting the location of the nearest phone if there’s no phone available in the lab.
Safeguards should be set around an unattended operation in the lab. If it is unavoidable to perform an unattended operation, lab users are asked to stick to the guidelines explained below.
For unattended operations involving highly hazardous materials, a light should be left on and an appropriate warning/explanation sign should be placed on the laboratory door, or in a conspicuous place that could be easily seen without putting someone else in danger in the event of an emergency.
Warning signs for unattended operations should include information regarding:
- The nature of the experiment.
- The chemicals used.
- Potential hazards (electrical, heat, etc.)
- The name of the person conducting the experiment and a contact number. A secondary name and contact number is also recommended.
It is important to consider potential risks of hazard that may take place when leaving an experiment unattended. Some precautions that may be taken include:
- Using secondary containments to prevent spills.
- Using safety shields and keeping the hood sash closed to contain chemicals and glass in case an explosion occurs.
- Removing idle chemicals or equipment or items that has the potential to react with the chemicals or other materials being used in the experiment.
- Using automatic shutoff devices to prevent accidents such as loss of cooling water shutoff, over-temperature shut off, etc.
- Using emergency power outlets for those pieces of equipment that could be negatively affected in the event electric service or other city utilities are interrupted.
Setting up procedures and policies for unattended operations is LS’s or responsible faculty member’s responsibility. Every lab user working under corresponding faculty member’s supervision should follow these procedures and policies.
Access to SU laboratories, workshops and other work areas housing hazardous materials or machinery is restricted to SU Faculty, staff, students, or other people on official business.
Necessary training is mandatory prior to laboratory access for all users. Access to laboratories is either through SU ID card or key system. Card activation is done for any particular laboratory through contact with LS. For all students, Responsible Faculty Member approval is required, apart from mandatory training. Laboratory keys can also be obtained from LS.
Visitors And Children In Labs
Non-lab members, particularly children under age of 16 are not allowed to access laboratories or other hazardous work places. Some exceptions to this rule are tours, open houses or university related business. LS and/or Responsible Faculty Member should authorize these instances. All children under age of 16 should be under supervision during lab visits.
Visiting Scientists And Other Similar Users
Some potential risks associated with visiting scientists and their usage of lab area and equipment includes: conflict of interest and intellectual property rights, physical injuries, and unwitting damage of property. Our faculty provides health and safety trainings to all lab users including visiting scientist prior to giving lab access to labs or equipment. Visiting scientists are asked to attend these trainings so that LS will provide lab/equipment access.
In case of a potential physical or health hazard incident, program chairperson, Responsible Faculty Member and LS are responsible of restriction of access of visitors or children to lab areas.
Pets in Labs
Pets are prohibited in laboratory facilities.
The chemical inventory should be searched and existing chemicals should be used prior to ordering any chemical. SU has an institutional subscription to the ChemWatch chemical inventory system that can help facilitate maintaining a chemical inventory. Everybody in the laboratory is obliged to attend ChemWatch Training and use it.
All purchased chemicals, with detailed specifications, amounts and placed location, must be reported to LS immediately upon arrival, so that they are registered to ChemWatch
Chemicals should be ordered in the smallest size possible that is necessary to carry out the experimental procedure. “Might be needed in the future” is an incorrect approach to order chemicals. Lab users should only order the amount they need.
There are several chemicals that require special approval to order. For those chemicals, please contact LS/LSS for further details. Note that building may restrict the amount of certain chemicals that can be stored in any room of the building. Further information can be obtained from ChemWatch.
Lab users should communicate with LS/LSS and Operational and Technical Services upon purchasing and installing large equipment, particularly those using faculty’s electric, water or gas services. It has to be made sure that faculty has sufficient sources to support this new piece of equipment. It should be noted faculty is not obliged to provide necessary infrastructure to install new purchased equipment. Lab users should communicate in advance with appropriate university units (such as Operational and Technical Services and LS/LSS) to determine and provide additional resources and potential issues so that building’s infrastructure is made ready for installation.
An example to this situation is the installation of fume hoods. As certain pieces of equipment such as fume hoods require special installation conditions and directly effects building ventilation system, lab users cannot decide alone and install these type of equipment with a third party contractor. They have to consult Operational and Technical Services and LS and LSS. Being proactive and consulting corresponding university units in advance is strongly encouraged.
For an equipment maintenance or repair lab users should consult Responsible Faculty Member or LS/LSS. These responsible people will take care of the paperwork with Operational and Technical Services and have the maintenance/repair process initiated. Faculty rules strictly forbid lab users repair utility services (electrical, plumbing, or gas issues) by themselves. Qualified personnel handle repairs only.
All chemicals, lab equipment and apparatus should be removed from hoods during maintenance or repair. Lab users must make sure that the work area is clean and free of hazardous chemicals. It is lab users’ responsibility to leave the hood safe for maintenance users and inform the users about potential hazards present around their working area.
In case of a lab occupancy change situation such as retiring of a faculty, new coming faculty member, new coming LS, graduating student or facility renovation, the occupant should inform her/his successor about potential issues and hazards.
Failure to address the change in occupancy can result in:
- Old, unlabelled chemicals, samples, or hazardous waste being left behind in refrigerators, freezers, and cabinets.
- Valuable furniture or equipment being moved or thrown away.
- Unknown chemical spills or contaminationbeing present.
These issues can result in costly remediation efforts and wasted resources for both the faculty and the university.
If you are planning to leave your laboratory or if you know of a research group or students that are planning to leave, there are a few simple steps that can be followed to ensure a smooth transition
- Notify your LS and LSS well in advance of the planned move.
- Ensure all chemical containers are properly labelled.
- Properly dispose of any hazardous and chemical waste left in the laboratory.
- Ensure all chemical spills and contamination has been cleaned up.
- The established checkout protocol (see Appendix 5.2.) is to be strictly followed by graduate students before leaving University Facilities.
Laboratory Design and Construction
Taking health and safety considerations is the key to provide best service during construction/renovation process. These measures should be taken in advance during the design process prior to construction process. Following information need to be given to LS and LSS before starting a new lab construction:
- Contact name, phone number, email
- Program, building and room(s) the project will occur in
- Expected start date for project
- The proposed equipment to be installed - fume hoods, biosafety cabinet, other capture devices, eyewash and emergency shower, toxic gas cabinet and monitoring devices, etc.
NOTE: A list of chemicals, including approximate usage (weekly/monthly) and storage quantities will be needed during the process to ensure proper ventilation rates and engineering controls.
Ventilation rates of the labs are determined with respect to number of people occupying the lab and type of research conducted. This is done as a measure of energy conservation. Lab users should notify LS and LSS if a new researcher comes to lab and function of the room changes, so that LS and LSS will check the ventilation rate.
A properly operating fume hood creates negative pressure in the lab. It removes more air from the room than is being supplied. On the contrary, a positive pressure is where more air being supplied to the room than is being removed. When this takes place, dirty air in the laboratory, which includes chemical vapor and dust, is blown outside of the lab into the halls. This can result in chemical odors permeating the hallways and surrounding rooms and may also negatively effect fume hood performance. Lab users should routinely check the air pressure of the lab and compare it to the hallway.
Laboratory users can perform a quick check of the air pressure in their labs by using modified version of the dry ice test procedure (see Appendix 4.1). If you discover your lab to be under positive air pressure, then please contact LSS for assistance.
Laboratories consume a lot of energy. The amount of energy consumed in a lab facility is much more than the energy use of an average non-lab academic building. This is mainly because there are multitude of heated and cooled, one-pass air for ventilation and fume hoods; electrically operated fans, specialized lab equipment; and large quantities of water and process-chilled water. In addition, some laboratories use large quantities of natural gas.
Technological advances in facility design resulted in considerable amount of energy savings in new constructed lab facilities. An example to this is computer controlled lab buildings. On the other hand, these energy saving initiatives are meaningful only when people in the lab help improve the energy conservation efforts.
Some of the very easy actions that may be taken to help reduce the energy consumption in the laboratory include:
- Turn off the lights when you leave the lab during the day or at the end of every day. Putting a setback (turn off themselves after a few minutes) is an efficient alternative to this.
- Before leaving the lab, make sure that you turned off all electrical devices if they’re idle.
- Use timers to turn other pieces of equipment on and off automatically.
- Turn off your computer's monitor when not in use. The monitor consumes over half of the energy used by the average computer. Turning your computer's energy saving features on is an alternative to that.
- Keep the sash closed on your fume hood, especially if you have a Variable Air Volume (VAV) type fume hood. This promotes both energy conservation and safety. Keeping your VAV hood sash closed can cut the air volume and cost by two thirds!
- Rooms that are too hot or too cool may be due to faulty thermostats or other controls that are malfunctioning or have drifted from set points, resulting in wasted energy as well as uncomfortable conditions for you. If you experience these problems, then contact LS or operational and technical services for assistance.
- Report drips of water from sink taps, chilled water connections or reverse osmosis (RO) faucets.
There is well-established laboratory health and safety standards to that regulate labs and other research areas. LSS conducts routine inspections in all research areas of the faculty to check if these regulations are applied.
Research areas are strongly encouraged to conduct their own self-inspections prior to LSS conducting an inspection of their research area to address any potential issues before the LSS inspection and to provide a training opportunity for researchers.
The main purpose of these inspections is to help Responsible Faculty Members and LS identify potential regulatory compliances or other problems that may affect lab users health and safety. This way, potential risks of health and safety hazards are also identified and unreasonable risks to both lab users and the rest of campus community are prevented. It is LSS’ responsibility to schedule such inspections.
Inspection checklist followed by LSS is provided in Appendix 5.3.
Self-inspections
Self-inspection system helps create a safety culture within the lab. It is an important part of lab safety regulations. It provides several benefits including:
- Raising the level of awareness of laboratory users.
- Determining the level of compliance with state and federal regulations.
- Identifying and eliminating potential risks prior to an official inspection carried out by government officials.
- Enabling lab specific training by identifying potential risks. Train the other lab users on these risks.
- Serving as a regular health and safety check of laboratory facilities.
- Serving as an outlet for Faculty, staff, and student concerns.
A self-inspection is recommended in following periods:
- On a daily basis in terms of housekeeping
- On a weekly basis by walk-throughs or collective clean-ups
- The ideal period for self-inspection is once in a month. For monthly inspection, an inspection checklist may be used. It is also recommended to ask Responsible Faculty Member or other researchers to join your monthly self-inspection.
- LSS is required in self-inspections at least once per semester. LSS utilizes her/his own inspection checklist.
There are multitude of benefits of regular self-inspection in terms of lab user’s health and safety. In addition to that, these inspections may also reduce legal liabilities since it is another chance to identify potential risks in the lab.
Inspections by Regulatory Agencies
These inspections can occur any time. In order to be prepared for official inspections, it is important to understand what regulations apply to laboratories. This handbook helps you to understand and apply these regulations in your laboratory. You can call LSS for any further information.
If inspector shows up in your work area unescorted, ask them to please wait and contact LSS immediately.
Laboratories need to take specific actions in order to provide security against theft of highly hazardous materials, valuable equipment, and to ensure compliance with government regulations. Each unit (programme and research group) is encouraged to review and develop procedures to ensure the security of all hazardous materials in their area of responsibility.
Each laboratory implements their own means of security, i.e. locking up controlled substances, syringes and needles, and radioactive materials. It is Responsible Faculty Member’s responsibility to assess the risks of a chemical and take security measures accordingly. The main purpose here is to prevent theft of dangerous chemicals. The easiest way to increase the security is to make sure that lab door is locked when you leave the unattended.
The Responsible Faculty Member is advised to determine the precautions necessary for the particular laboratory, and the determined set of rules is to be followed by all users in this laboratory.
Security Guidelines
Security guidelines are to eliminate the risk of removal of any hazardous material from laboratory. These include:
- Note that, although they are related, laboratory security is different from laboratory safety. The main purpose of lab security is to protect hazardous chemicals.
- Access to the areas where hazardous chemicals are used and stored should be controlled and limited. Limitation may also include some off-hours or granting access only to lab users authorized by Responsible Faculty Member.
- Freezers, refrigerators, storage cabinets, and other containers of biological agents, hazardous chemicals and radioactive materials should always be kept locked.
- Hazardous materials should always be secured and never be left unattended. The most important precaution to this is to lock laboratory door when unattended.
- Note: If users work alone and use the buddy system with someone outside of the research group, allowing access for that individual will need to be addressed prior to the initiation of working alone.
- Be aware of who is in the laboratory at all times. Approach anyone who you don't recognize and appears to be wandering in laboratory areas and hallways and ask if you can help direct them.
- A log sheet system may be used to secure highly hazardous chemicals. A periodic inventory check of hazardous chemicals is strongly advised. Any missing chemical should be immediately reported to LSS.
- Follow new chemicals ordering procedure; be aware of what new chemicals will be brought to your lab area. Packages of potentially infectious materials are only opened in biological cabinets.
- Use emergency plan for reporting incidents. Be sure to include the lab's emergency contact information on located on or near your laboratory door.
- Be aware of the classes of security risks of hazardous chemicals. Laboratory users should be aware of the highly hazardous materials or other special materials of concern.
Pay special attention to the following:
- Common labs
- Unrestricted access to toxic chemicals
- Unlocked support rooms
- Toxic gas security
- Unsecured biological materials and waste
- Access to controlled substances
- Changes in chemical inventory
- Storeroom security
- Chemical waste collection areas
- Unusual activities
Many of the laboratory supply catalogs carry information and products such as various locks, lock boxes, and other security devices for chemical storage in laboratories. For more information, you can contact LS/LSS for assistance or consult with the Operational and Technical Services about security devices.
References
University of Cornell, Laboratory Safety Manual and Chemical Hygiene Plan, Chapter 4, Administrative Controls (2015, March). Retrieved from https://sp.ehs.cornell.edu/lab-research-safety/laboratory-safety-manual/Pages/ch4.aspx
Biological Safety Levels
Detaylı BilgiGel Room, Dark Room, Radioisotope Room And Cold Room Safety
Detaylı BilgiDecontamination
Detaylı BilgiSafety data sheets (SDS), for chemical products have been available to lab users for many years. However because many laboratory users, whether in research, public health, teaching, etc., are exposed to not only chemicals but infectious substances as well, there was a large gap in the readily available safety literature for lab members. These SDS are produced for personnel working in the life sciences as quick safety reference material relating to infectious micro-organisms. Sabanci University employs ChemWatch software for SDS reference documents generation.
The SDS are organized to contain health hazard information such as infectious dose, viability (including decontamination), medical information, laboratory hazard, recommended precautions, handling information and spill procedures. The intent of these documents is to provide a safety resource for laboratory users working with these infectious substances. Because these users are usually working in a scientific setting and are potentially exposed to much higher concentrations of these human pathogens than the general public, the terminology in these SDS is technical and detailed, containing information that is relevant specifically to the laboratory setting. It is hoped along with good laboratory practices, these SDS will help provide a safer, healthier environment for everyone
Emergencies, Exposures And Spills
Detaylı BilgiPersonal Protective Equipment
Detaylı BilgiTransportation And Shipment Of Biological Materials
Detaylı BilgiDue to the extreme low temperature and high rate of gas escape, a number of precaution and safety measures must be applied while working with cryogenic liquids.
Extreme Cold Hazard
Brief exposures that would not affect skin on the face or hands can damage delicate tissues such as the eyes. Prolonged exposure of the skin or contact with cold surfaces can cause frostbite.
When a tissue freezes, one may not feel pain initially. An intense pain is felt upon thawing. If the skin is unprotected it can stick to metal containers of cryogenic liquids. It may then tear when pulled away. Even non-metallic materials are dangerous to touch at low temperature.
Breathing of extremely cold air for long periods of time may damage the lungs.
Due to low temperature, many common materials such as carbon steel, rubber and plastics may become brittle upon contact with cryogenic liquids and easily break under stress.
Asphyxiation Hazard
Normally, 21% of air is composed of oxygen. Symptoms of asphyxia develop when the oxygen percentage of air drops to 15-16%. At 12% oxygen, the individual will lose consciousness without warning and may be unaware of any danger. Cryogenic liquids produce a large amount of gas upon vaporization. One volume of liquid nitrogen vaporizes to yield 694 equal volumes of nitrogen gas at standard conditions. If the oxygen level is not regulated, asphyxiation may quickly lead to death. The gaseous product formed by cryogenic liquid evaporation is very cold and heavier than air. This gas does not disperse in air, but accumulate near the floor. Although this gas is non-toxic, it replaces the air. Thus, oxygen deficiency becomes a serious hazard risk closed spaces. Therefore, cryogenic liquids should only be used and stored in well-ventilated areas.
Symptoms of asphyxiation include giddiness, mental confusion, loss of judgement, loss of coordination, weakness, and nausea, fainting and lead to death. A few breaths of oxygen-deficient air are enough to reduce the dissolved oxygen level in blood. Once blood oxygen level is dropped, mental failure and coma follow very quickly. Asphyxiation symptoms may not always be observed, even though they do, loss of mental abilities and coordination may make it impossible for people to ask for help.
Gaseous products of most cryogens are odorless, colorless and tasteless. In addition, most cryogenic liquids are colorless, except oxygen (light-blue). The most significant warning property of extremely low liquids and their vapors is that they tend to form visible fog by condensing the moisture around. In other words, a fog cloud does not define the vapor cloud but it rather defines the area where vapor is still cold enough to condense the moisture in the air. Although fog cloud is an important clue to cryogenic gas leak, it is highly probable that the leak extends beyond the fog cloud. Therefore, fog cloud must never be used to define the leak area and should never be entered by anyone.
In summary, cryogenic vapors are undetectable to human sensory system. Therefore, one should not enter a possible oxygen-deflected area without wearing an external breathing air source and without carrying and atmospheric monitor. It is strongly advised to check the safe oxygen level prior to enter these areas.
Oxygen Enriched Air
Liquid oxygen in an enclosed area vaporizes to increase the amount of oxygen in air and saturate the combustibles, such as clothing. This may easily start a fire if an ignition source is present. Oxygen is not flammable, but it starts and accelerates combustion reactions.
Release of liquids below the boiling point of air may condense the surrounding air that leads to an oxygen-enriched local atmosphere. Moreover, extremely cold cryogens, such as helium, may even freeze the surrounding air.
Explosion Due to Rapid Expansion
If a cryogenic liquid vaporizes more than expected in a sealed container, it increases the internal pressure of container, which may lead to a rupture in the container. Therefore, pressurized cryogenic containers are designed in multiple protection devices to prevent over-pressure. A pressure relief device must be installed to prevent liquid trapping. Extra care must be taken while using pressurized containers. Please make sure that you have switched off the valve completely after use.
Special Helium Precautions
The most critical safety concern in dealing with liquid helium is its extremely low temperature. Helium is so cold that once it released it freezes all gases around. This includes not only water, but also nitrogen and oxygen; freezing of these gases inside a Dewar or a pipeline cause an “ice” plug which may lead to closing up the neck and create a bomb. Therefore, it is of utmost importance the set up a procedure for helium usage and to follow it strictly. This helps prevent other gases, including air, from entering the liquid helium delivery lines. If you suspect a blockage, remove the Dewar to a safe place and immediately contact the vendor. If helium is transferred by a piping system without vacuum jacket, air surrounding the pipe may liquefy. After condensation, nitrogen evaporates first, leaving an oxygen-enriched air around the pipe. This area, where the liquid is collected, should be insulated and oxygen-compatible.
Storage and Use
All cryogenic liquids must be stored and used in a well-ventilated area.
- Dewars: Dewars should be selected as non-pressurized, vacuum-walled containers. They should be equipped with a loose-fitting cap or open top and should store only small amounts of liquid.
- Cryogenic Liquid Cylinders: These are sealed, vacuum-walled containers, which do contain pressure up to 350 psig. Cryogenic liquids can also be extracted from these containers.
- Cryogenic Storage Tanks: These tanks range in size from 1900 to 4.5 x 105 L and are always pad mounted. Liquid and gas can be extracted from these containers.
Personal Safety
Note that eyes are the most sensitive part of body against extreme cold liquid or vapors.
To handle cryogens, it is recommended to use personal protective equipment (PPE) including full-face shield over safety glasses, loose-fitting thermal insulated or leather gloves, long sleeved shirts and trousers without cuffs. To allow quick removal in case of a spill, gloves should be in loose fit. Note that cryogenic gloves does not allow you to immerse your hand inside a cryogenic liquid, they are only designed for and provide a short-term protection, particularly for accidental contacts. While transferring cryogenic liquids, you should not wear jewellery rings watches etc.
Safety Practices
Cryogenic liquids must be handled, stored and used only in containers or systems designed in applicable standards, procedures or proven safe practices.
- To withstand the extreme low temperatures, all the components including piping, valves etc. should be made up of appropriate materials that endure to low temperatures.
- To prevent over-pressure, pressure relief systems should be used in piping.
- Cryogenic containers or any part of these systems that could be valved off should be equipped with a pressure relief valve. These valves should be positioned to face forward.
- During transfer operations including opening cryogenic containers all required PPE should be worn. People must move slowly during transfer. Note that extra care must be taken not to contact non-insulated pipes and other components.
- Open transfers are allowed only in well-ventilated areas.
- Never use a funnel while transferring cryogenic liquids.
- Tongs or other similar devices should be used to immerse and remove objects from cryogenic liquids.
- Hazard reviews are required on all newly purchased, built or modified tools using cryogenic materials.
Frostbite
In case of a skin contact with cryogenic liquid, all the clothing that may restrict the blood circulation should be removed from affected area. Frozen area should never be rubbed as it may result in tissue damage. If practical, affected area should be placed in a water bath with temperature not exceeding 40 °C. Dry heaters should never be used for frostbite. Call Health Center (7666) as soon as possible. Frozen tissue is usually pain-free. Symptoms of freezing are a waxy appearance and a possible yellow color. Once it is thawed, it is prone to swelling, pain and infection. If thawing has started, cover the affected area with a sterile dressing and seek medical attendance. If the exposure is massive, victim’s clothes should be removed while showering with warm water. Call Health Center (7666) immediately. In case of eye exposure to cryogenics, warm the frostbite area immediately with water with temperature not exceeding 40 °C. Seek medical attention. Should the body temperature depressed, warm the victim slowly and gradually. Be aware of the shock risk upon hypothermia correction. Cardiac dysrhythmia may be associated with severe hypothermia.
Asphyxiation
Victims exposed to oxygen-deficient air should be immediately moved a normal atmosphere. Artificial respiration should be administrated if the victim does not inhale. If available, administer supplementary oxygen with respirator.
Emergency Procedures
Note that there are no visible evidences of oxygen-deficient atmospheres. They don’t have warning properties. Always carry an air source while entering a suspected oxygen-deficient area. Carry air-monitoring devices to measure oxygen levels on site. When it is necessary to work in an oxygen-deficient area, supplied air must be provided. Should a Dewar of cryogenic liquid be venting continuously, call LS/LSS immediately. For further information please see Emergency Procedures Section.
Detaylı BilgiClean Room Safety
In general, the classifications of cleanrooms are done depending on the number and size of particles permitted per volume of air. Numbers like "class 100" or "class 1000" denote the number of particles of size 0.5 mm or larger permitted per cubic foot of air.
The classifications of cleanrooms are done with two standards; (1) US Federal Standard 209E and (2) the newer standard TC 209 from the International Standards Organization. The number of particles found in the laboratory’s air is a basis for these two standards used in classification of cleanrooms. As an example; Sabancı University lab has been classified as a class 10 cleanroom because there are less than 10 particles per cubic foot. Table 11.1 relates FS to ISO classifications:
| Table11.1 ISO 14644-1 Cleanroom Standards | |||||||
|---|---|---|---|---|---|---|---|
| Class | maximum particles/m3 | FED STD 209E equivalent | |||||
| >=0.1 µm | >=0.2 µm | >=0.3 µm | >=0.5 µm | >=1 µm | >=5 µm | ||
| ISO 1 | 10 | 2 | |||||
| ISO 2 | 100 | 24 | 10 | 4 | |||
| ISO 3 | 1,000 | 237 | 102 | 35 | 8 | Class 1 | |
| ISO 4 | 10,000 | 2,370 | 1,020 | 352 | 83 | Class 10 | |
| ISO 5 | 100,000 | 23,700 | 10,200 | 3,520 | 832 | 29 | Class 100 |
| ISO 6 | 1,000,000 | 237,000 | 102,000 | 35,200 | 8,320 | 293 | Class 1,000 |
| ISO 7 | 352,000 | 83,200 | 2,930 | Class 10,000 | |||
| ISO 8 | 3,520,000 | 832,000 | 29,300 | Class 100,000 | |||
| ISO 9 | 35,200,000 | 8,320,000 | 293,000 | Room Air | |||
* Table adopted from Brigham Young University, Department of Electrical and Computer Engineering, Cleanroom Classification (Reference 11.1).
In the K 10,000 two situations exist:
- Mo - Fri, 07.00 a.m.. - 06.00 p.m. air extraction high
- Mo - Fri, 06.00 p.m.. - 07.00 a.m., and on Saturday, Sunday and on holidays: air extraction low
Air extraction high: conditions meet safety and minimum contamination level requirements.
Air extraction low: conditions meet safety requirements; minimum contamination level not guaranteed.
Proper gowning is an important part of cleanroom maintenance and serves to prevent, or at least minimize the contaminations and particle formation arising from human body and clothing. Human can be considered as the “dirtiest” object in a cleanroom and the main source of contamination. Recall that the airflow in a cleanroom is downwards i.e., from ceiling to floor. Thus, floor is the dirtiest place and is where most particles are found. Thus, gowning steps (listed below) should be from top to bottom, while de-gowning is in reverse order:
- Remove your coat or jacket before entering the cleanroom. There’s a coat rack available at the corner of cleanroom entrance. Personal items should not be carried into cleanroom. Also, if you are wearing woollen clothes you should remove them before entering the cleanroom in order to prevent electrostatic discharge (ESD) to your work.
- Once you enter cleanroom entry room, put on a pair of shoe covers. This is done by wearing one shoe cover and swinging the leg over the bench, then covering the other shoe and swinging that as well. You are not allowed to wear uncovered shoes beyond this point.
- Wear cleanroom hood. Your hair should be fully covered with hood.
- Wear the coverall, tuck in hood flaps and zip all the way.
- Put on boots over legs of coverall.
- Put on safety glasses or goggles. Since chemicals may permeate contact lenses and adsorbed on them, contact lenses are not recommended to use in cleanroom. Personal glasses including prescription lenses offer sufficient protection.
- Wear vinyl gloves. Care should be taken not to touch the outside of gloves with bare hands. Pull the gloves over sleeve of coverall.
Once you carry out all the steps in turn, you are ready to enter the cleanroom. Without gloves you shouldn’t touch the door handle, or anything beyond it. The gloves, along with blue inner shoe cover are disposable and therefore should be disposed after use. The garment, on the other hand, can be worn several times. If your gloves are teared or dirty, you can replace them inside cleanroom.
As personal safety measures state, shorts, skirts and open-toed (or open-sided, open-heeled) shoes of any kind should not be worn in cleanroom. It is advised to keep another pair of lab shoes or pants available and worn prior to entering cleanroom. There are lockers available at the entrance of cleanroom. You may request a locker from LS.
In addition, visitors coming with you on an approved cleanroom tour should also follow all the clothing rules of cleanroom and are not allowed to enter cleanroom without wearing the clothes mentioned above. Registered cleanroom user is the main responsible to make sure that visitor is appropriately attired before entering. There will be no exceptions to this rule. Cooperation of all lab users is highly appreciated.
MEL LS should approve a cleanroom lab tour. Requests for cleanroom tour are made in advance. Size of the tour group should not exceed 3 users. For group tours with more than 3 person, please consult to LS.
Working alone is prohibited for research purposes, particularly involving hazardous chemicals and experimental procedures. Otherwise, LS or Responsible Faculty Member should prepare guidelines and standard operating procedures (see Standart Operating Procedures section) highlighting working alone principles, notifying procedures and instances when working alone is strictly forbidden. In cases where working alone is a must, LS/LSS or Responsible Faculty Member should approve the work and experimental setup. Whole procedure should be monitored by surveillance system.
It is strongly advisable to notify someone in the working area, in the next room or in the same floor. A “buddy system” may be established where buddy person performs routine checks on alone users periodically. This is to make sure that a safe experimental procedure is followed. Routine checks may be carried out either by physically or via a phone. A buddy should not be in the same room when the person working alone is carrying out a highly hazardous work. Visual check systems is another option to make sure that working alone is being performed safely or to determine if help is needed.
If an emergency event takes place that involves highly hazardous chemicals and buddy had to leave the lab before the end of experimental procedure, the buddy should notify security (7555). Notification includes name of the person working alone, location of the accident and the time of accident. In addition, buddy warns the person conducting the experiment involving hazardous chemicals so that the person can finish the experiment in the safest manner possible and inform security personnel on the latest condition of the experiment. It is strictly forbidden to use security personnel as a buddy. This will impair the safety of all the people involved.
Please note: For rooms that are locked due to security needs, prior arrangements are required to allow the designated buddy access. It is also important to understand that if the door to the lab does not have a window, or if the window is covered, then there is a chance that if something happened to a person working alone in a locked lab, then they may not be discovered until someone else from the lab goes into the room (which could be a day or more later).
Instances when working alone is permitted includes:
- Office work
- Housekeeping activities
- Assembly or modification of laboratory apparatus. Here, no chemical, electrical, or other physical hazards should be present.
- Routine lab functions as a part of a standard operating procedure.
Instances when a “buddy system” is needed includes:
- Toxic or otherwise hazardous chemicals included experiments
- Experiments involving high-pressure equipment
- Experiments involving large quantities of cryogenic materials
- Experiments involving work with unstable (explosives) materials
- Experiments involving Class 3 or 4 Lasers
- Transfer of large quantities of flammable materials, acids, bases, and other hazardous materials
- Changing out compressed gas cylinders containing hazardous materials
Setting up procedures and policies for working alone is LS’s or Responsible Faculty Member’s responsibility. Every lab user working under the corresponding faculty member’s supervision should follow these procedures and policies.
- Wearing appropriate protective equipment (safety glasses, gloves, etc.) is mandatory.
- While working in the laminar flow of the clean benches, keep clear of exposure by inhalation of vapor. Do not put your head inside the fume cupboard.
- Once you pass the test, you are allowed to work with chemicals independently.
- When the air extraction is lower than usual (06.00 p.m. - 07.00 a.m.), it is not allowed to:
- work with chemicals alone. There must be an experienced person working with you.
- work with HF, bromine and peroxide solutions.
- HF: Immediately rinse with lage amounts of water. Alert the LS/LSS to apply first aid with the HF emergency kit.
- Acids & alkalines: Immediately rinse with Diphoterine spray bottle; contact LS/LSS afterwards.
- Organic liquids: Immediately rinse with Diphoterine spray bottle; contact LS/LSS afterwards.
- Strong acids: Call LS/LSS.
- Organic liquids: Quickly absorb the liquid with tissues/absorbant mats.
- When you finished your experiments, do not leave chemical residues. Everything, including the workspace, should be cleaned after use.
- Hands should be washed thoroughly with soap and water after you leave the cleanroom.
- Due to potential fire risk, organic solvent should not be placed anywhere close to hot plates. Organic solvents are only allowed to be heated in a water or oil bath.
- Recall that water should never be added on top of acids or bases.
- Since they are incompatible, organic liquids should be stored away from acids, peroxides and alkalines. They may give rise to an explosion.
- Always report the spills by calling the LS/LSS.
- When chemicals spilt on the body, then with:
- When chemicals spilt outside the workbench then with:
Procedures to follow in the case of a calamity are explained in the "emergency procedures" section.
- Please schedule your runs in advance when possible. Do not sign up for more time than you think you may need.
- Comply with your schedule. If you do not log into the equipment within 15 minutes after your time begins, you will lose your time and other users may use the equipment.
- Remove tooling (boats, sample holders, etc.) from systems and clean-up particles from process chamber. Note any anomalies to the staff and next user.
NOTE: To establish a safe use of equipment, all approved MEL users are required in the cleanroom during the operation of equipment. Exceptions to this rule are furnace tubes because a deposition may take extended time periods.
- Necessary clothing should be worn properly. See Protective Clothing section.
- Gloves should be worn all the time including clean bench use, lithography experiments and loading/unloading reactors.
- There are designated cleanroom papers available to make notes. Normal paper is only allowed in cleanroom if it is sealed in plastic. It is strictly forbidden to use any kind of wood, cardboard or packing materials.
- Minimize the amount of items to take into cleanroom with you. Substrate boxes, syringes, ball pen or felt pen are allowed, but pencils are strictly forbidden.
- While working with benches, keep your head outside.
- Redundant clothes (jacket, jumper, etc.) and bags are not allowed in cleanroom. They should be kept in wardrobes at the entrance of cleanroom. Valuable items can also be stored in lockers.
- Avoid any turbulence: Do not hurry; use minimal working area on a clean bench, but stay in the centre of the down flow.
Cleanroom evacuation reasons are listed below. If one of the conditions listed takes place while you are working in the cleanroom, you should warn other people in the cleanroom so that they can evacuate and help precede the protocols. Reasons to evacuate cleanroom include:
- Power loss: In this case, do not pull the fire alarm.
- Fire: You should pull the fire alarm and hazard gas shut-off button.
- Hazardous gas leak: In this case you should engage the hazard gas shut-off button.
- Major chemical spill outside a chemical hood, such as breaking a chemical bottle
- Unconscious lab user: You should notify other lab users.
- Non gaseous chemical exposure
- Physical injury
- Medical emergency
- Loss of fume hood exhaust
Please conduct the following procedure in case of an emergency event listed above:
- Immediately stop what you are doing, aborting your process.
- Quickly proceed to the closest emergency exit available. Do not run unless there is a life-threatening situation.
- Engage the hazardous gas shut-off button while evacuating cleanroom. Trigger the fire alarm in case of fire.
- Make sure that other people are evacuating cleanroom. If there are people not able to exit by her/his own, call security (7555) to help them. Do not lose time to remove cleanroom garments.
- Once you are out of cleanroom, proceed to the first floor exit of FENS building. Call LSS and report them about the incident.
- If they are not present in the event of evacuation, notify MEL LS.
It is not necessary to evacuate cleanroom in cases of:
- Small chemical spill inside a hood
- Inert gas leak
Next sections focus on approved and non-approved behaviour while using cleanroom. As a rule of thumb, if you are not sure whether or not you are allowed to do something, DON’T!
Cleanroom Conduct-General Behaviour
- If you see, hear or smell something unusual, use common sense and take necessary action.
- Adopt a “better safe than sorry” attitude.
- Report any violations of the rules to LS.
- Report equipment problem, malfunction, or breakage to appropriate LS.
- Hood, gown, booties with shoe covers, masks, gloves, and safety eyewear. Bouffant caps are required for long hair.
- Keep all hair and ears covered with hood or cap.
- Never open your gown in the cleanroom.
- No corrugated cardboard, styrofoam, foam rubber or non-cleanroom paper is permitted in the cleanroom.
- Be considerate by cleaning up your own mess, not messing up someone else's work.
- Let the LS know when new supplies are needed, etc.
- No pencil, erasers or retractable pens are permitted in the cleanroom.
- Only authorized users may enter the cleanroom unescorted.
- No eating and drinking is permitted in the lab.
- Smoking is not permitted in the lab.
- Ask for permission before bringing anything in or taking anything out of the cleanroom.
- Do not run in the lab.
- DO NOT modify equipment without the approval of the LS.
- Do not cover up any equipment problem or breakage. This will only make the situation worse.
- If you are not sure how a system works or anything else about it, ask the responsible person before you use it and in case something worse happens. Please do not be ashamed or hesitate to ask.
- Hair should always be inside the hood/mask.
- Tacky mats in the entrance of cleanroom help decrease the dirt.
- Gloves should be replaced if they are dirty.
- Only pens and paper provided by cleanroom staff should be used.
- Make sure that you leave your working space cleaner than you found.
- Any material brought to cleanroom should be cleaned with isopropanol (IPA) and cleanroom wipe prior to enter cleanroom. There is IPA available at the entry terminal. Note that LS should approve any item intended to brought to cleanroom beforehand. An exception to this rule is wafer boxes and holders.
- Gloves should be worn all the time. Do not touch anything without gloves.
- Once you wear your gloves do not contaminate them by touching your skin, face or hair.
- Do not bring paper or cardboard into the lab.
- Do not transfer quartz-ware or Teflon-ware between equipment, particularly the thin film furnaces.
- Do not deviate from your approved process sequence.
Teflon-ware can only be used in a designated hood. Teflon tanks or graduated cylinders cannot be transferred between hoods. Being aware of that the possibility of chemical contamination is low, LS attempts to minimize the risks arising from these kinds of occurrences. In addition, Teflon wafer boats for wet chemical processing should not be removed from their designated area. In other words, when you have successfully rinsed and dried following a chemical process, you should transfer the process wafers to your process box (usually a black process box) and then remove them from the fabrication medium.
- Vacuum pens or wafer tweezers are used to handle wafers.
- If applicable, “dump” transfers are done from-cassette-to-cassette.
- “In process” wafers can be stored by sealing and labelling. Keep them either in storage bins or in a dry-box.
- Conduct appropriate labelling, i.e. your name, PI’s name, run name/number, date started, your phone number. Note that unlabelled wafers are subject to discard.
- Broken wafers in the container can be discarded. You may consult LS for disposal procedure. Wafers or any part of them are not discarded in common waste. Note that this violates Sabancı University FENS standards and may result in injury of you or others.
- Never touch wafers with hand or using tweezers.
- Never conduct your process with a broken or partially broken wafer*.
- Do not breathe on wafers.
* Permission of LS is required for processing partial wafers. Before starting a process with a partial wafer or with wafer different than the standard sizes (smaller or larger than 100 mm (4”)) you should ask and receive approval. Please be aware that resources of MEL are limited assisting such fabrications and processing.
- Chemicals should be poured slowly and carefully. Note that acids are added to water upon mixing.
- Wear necessary personal protective equipment, including face shield, chemical-resistant gloves, sleeve guards, and an apron while pouring chemicals.
- Chemicals should be transported, stored and disposed of carefully.
- You should know the location of first aid kits, eyewashes and SDS’s. Note that FENS provides an online SDS tracking software called as ChemWatch.
- Prior to disposal, you should rinse acid bottles at least 3 times in the hood where it is used. Empty acid bottles should be stored in boxes near acid cabinet. LS regularly sends these bottles to waste. Plastic bottles may be disposed of in the recyle bins.
- While lift-off processes, organic acids are allowed in metal etching. But they should not be used is acid/base metal etching is being carried out.
- Organics solvents, particularly acetone, and their designated area to use are separated metal etching area. Cleaning of glassware should be carried out at this designated area.
- All organic solvent bottles should be returned to solvent storage cabinet after use. In addition, organic solvents in squirt bottles should also be stored in solvent hood.
- Note that the primary sources of exposure to chemicals are:
- Skin- Absorption
- Lungs- Inhalation (the fastest route for chemicals to enter the bloodstream)
- Mouth- Ingestion
- Chemical bottles should not be opened outside of a fume hood.
- You should not use chemicals you are not familiar with unless you review their SDS.
- Unlabelled chemicals should not be used.
- Solvents and acids should never be mixed or used in the same working area. Note that this mixture may lead to an explosion.
- Colorless drops may not be water. Do not assume they are water drops and act accordingly.
- Chemicals should not be disposed of via drain.
- Containers of chemicals should not be stored in hoods.
- Only approved carts should be used to transport gas cylinders.
- You should be aware of and take precautions regarding risks of hazards related to gases to be used.
- Gas cylinders should be secured all the time.
- You should be aware of the locations of emergency gas shut-off buttons, emergency exits and fire extinguishers. You should also know emergency evacuation procedures regarding gases.
- Odor is not a detection method for possible toxic gas leaks. Do not try to detect a gas leak by odor.
- Inert gases are hazardous. Do not assume otherwise.
The list of the compressed gases used in MEL cleanroom is shown in Table 11.2.
| Table 11.2 List of compressed gases used in MEL cleanroom | ||
|---|---|---|
| Name | Formula | Hazard Statement |
| Argon | Ar | May cause drowsiness or dizziness |
| Nitrogen | N2 | May cause respiratory irritation, drowsiness or dizziness |
| Oxygen | O2 | May cause or intensify fire; oxidizer, may cause respiratory irritation |
| Sulfur Hexafluoride | SF6 | May cause drowsiness or dizziness |
| Tetrafluoromethane | CHF4 | May cause drowsiness or dizziness |
Notes:
- Inert and non-flammable gases, although non-toxic, can cause asphyxiation (suffocation) by displacing oxygen.
- Pyrophoric means spontaneously combustible in contact with air.
- Any gas not labeled properly should be considered toxic.
- Loss of nitrogen to turbo-pumps or cryo-pumps may result in pump in the buildup of toxic waste. Under no circumstances should non-MEL staff attempt any repair or restorative action on vacuum pumps associated with MEL equipment.
- Safety data sheet (SDS) are available for review at any time. Please contact LSS for any safety information.
Emergency Procedures
Note that there are no visible evidences of oxygen-deficient atmospheres. They don’t have warning properties. Always carry an air source while entering a suspected oxygen-deficient area. Carry air-monitoring devices to measure oxygen levels on site. When it is necessary to work in an oxygen-deficient area, supplied air must be provided. Should a Dewar of cryogenic liquid be venting continuously, call LS/LSS immediately. For further information please see Emergency Procedures Section.
Detaylı bilgiRadioactive Waste Management
Detaylı bilgiNMR device is operated by a dedicated specialist and the device and its components are not to be handled or removed without any permission under any circumstance. The following text is only intended to inform you about the device
Superconducting Magnet
A superconducting magnet is an electromagnet that is built using superconducting coils.
Our 500 MHz magnet system comprises of a fully persistent 11.74 Tesla magnet which is immersed in a bath of liquid helium (-269 °C) at which temperature the resistance of the coil is zero; once energized the magnet can run continuously.
The helium vessel is shielded from room temperature by a vessel filled with liquid nitrogen (-196 °C) and an outer vacuum to keep the liquid helium from rapidly boiling off.
The liquid helium and liquid nitrogen are not pressurized but are allowed to boil-off over a number of weeks; liquid helium and nitrogen must be added periodically.
The magnet is always on.
Magnetic Field Strength
Magnet strength is described in terms of Gauss or Tesla units (1 T = 10,000 G).
- Earth’s magnetic field: 0.6 Gauss at the equator
- Small bar magnet: 100 Gauss
- Refrigerator magnet: 100 - 150 Gauss
- MRI medical scanners: 0.3 - 1.5 Tesla (3 - 15,000 G)
High magnetic field NMR spectrometers and other superconducting magnets:
- 200 MHz: 4.7 Tesla (47,000 G)
- 300 MHz: 7.0 Tesla (70,000 G)
- 500 MHz: 11.7 Tesla (117,000 G)
- 800 MHz: 18.8 Tesla (181,000 G)
Surface of a neutron star: 1x108 Tesla (1012 Gauss)
Warning
Exposure to strong magnetic fields can cause serious injury or death and significant damage to personal property, equipment and data.
Nmr Safety
- 5-gauss perimeter should be minded by individuals with medical devices (e.g. cardiac pacemakers and metal prostheses). People using pacemakers, prosthetic parts and metal blood vessel clips should contact their physicians about the possible health risks before entering the NMR room because of the fact that the NMR spectrometers generate strong magnetic fields.
- Strong magnetic fields surrounding the NMR spectrometers can damage floppy disks, tapes, cards with magnetic strips, cellular phones, laptops and mechanical watches, so they should remain outside the 5-Gauss perimeter.
- Strong magnetic fields attract objects containing steel, iron, and other ferromagnetic materials (i.e., electronic equipment, compressed gas cylinders, steel chairs, and steel carts), so they should remain outside the 5-gauss perimeter. Personal injury and extensive damage to the probe, Dewar, and superconducting solenoid may be caused as these objects can fly toward the magnet. Only non-ferromagnetic materials are allowed to be used near the instruments.
- The magnet/Dewar has a high center of gravity and may collapse in an earthquake or if struck by a large object. People near the magnet may face serious injuries and the sudden release of nitrogen and helium gases from the Dewar will displace breathable oxygen in the room. The instruments are supported by anti-vibration legs that are attached to the floor.
- In the event of a "magnet quench" (sudden release of gases from the Dewar), leave the room immediately and contact NMR LS. Rapid expansion of liquid helium or nitrogen gas can displace breathable oxygen in the room creating the possibility of asphyxiation. Do not re-enter the room until the oxygen level has returned to normal. See specific “Cryogenic Purge – Quench” instructions.
- Handling cryogens is dangerous and can cause serious burns. Therefore, only individuals who have had special training and wear safety glasses, gloves and closed toed shoes should transfer liquid helium and nitrogen to the instruments.
- If you’re performing a temperature change experiment, always wear safety glasses near the magnet. Sample subjected to a temperature change can build up excessive pressure which can break the sample tube and broken glass and hot or toxic chemicals can cause injury. To avoid this hazard, establish the freezing and boiling points of a sample and never rapidly heat or cool a sample.
- Extra caution should be provided about the sample tubes as they are fragile and break easily. Removing the probe, the top of the sample tube can break off. The sample should be ejected before removing the probe from the magnet. Use extreme caution when removing the probe if the sample cannot be ejected.
- In the presence of flammable gases or fumes, NMR spectrometers should not be operated as there is risk of injury or death from inhalation, fire and explosion created by flammable gases or fumes.
- If a probe is in place, looking down the barrel of an NMR spectrometer should be avoided. Pneumatic ejection of a sample from the probe could cause injury.

In our lab, warning signs (Figure 15.1) and colored bands are displayed in areas where the field exceeds 5 Gauss; the location of the 10 Gauss region is located slightly inside the 5 Gauss region:
10 GAUSS WARNING - between RED and ORANGE BAND (Figure 15.2) STRONG MAGNETIC FIELD
Figure 15.1 Warning sign
Pacemaker, Metallic Implant hazard
Strong magnetic and RF fields can cause serious injury that may result in death of people with implanted or attached medical devices, such as pacemakers and prosthetic parts.
Such people must not go closer to the magnet than this sign until safety at a closer distance is identified by a physician or device manufactures.
Magnetic Media, ATM/Credit Cards
Strong magnetic and RF fields are present that can erase magnetic media,
disable ATM and credit cards and damage some watches. Do not take such objects closer to the magnet than this sign.
5 GAUSS WARNING – between red and green band (Figure 15.2.b)
STRONG MAGNETIC FIELD
Tools and Equipment
Strong magnetic and radio frequency fields are present that can cause magnetic items suddenly fly toward the magnet, which could cause personal injury or serious damage.
Do not take tools, equipment or personal items containing steel, iron or other magnetic materials closer to the magnet than this sign.
The warnings at the NMR Lab door are shown below in Figure 15.2.a:

Cryogenic Purge – Quench
Detaylı bilgiOther Hazards
Detaylı bilgiFume Hood Inspection and Testing Program
Detaylı bilgiOther Capture Or Containment Devices
Detaylı bilgiAir pressure in research laboratories should be negative to the hallways and offices. When positive air pressure occurs, more air is being supplied to the room than what is being removed by the fume hood or general exhaust. This can result in air from the laboratory (including chemical vapors and dusts) being blown out into the hallway outside of the lab and chemical odors permeating the hallways and surrounding rooms.
Ensure the entrance door to the laboratory is closed.
If you notice odors that seem to be escaping from a lab, then please contact LS/LSS for assistance.
Water Protection
Water supply connected of feeded equipment or laboratory apparatus uses backflow protection or is connected to a faucet with a vacuum breaker. Backflow prevention and vacuum breakers for water used experimental process or with a piece of equipment provides protection against back flowing or and contaminating the laboratory’s and building’s water supply system. Chemical contamination and/or temperature extremes could be observed in case of improper backflow prevention
Common water protection problems encountered in labs are listed below:
- A tube attached to a faucet without a vacuum breaker,
- Improper tubing connections causing leakage,
- Drainage tubing hanging down into the sink.
These tubes can be immersed in wash water when the sink is stopped up and backflow into the faucet, contaminating the building’s water supply.
References and sources for information from the relevant websites and documentation of different universities, NGOs and government agencies used in the preparation of this website are provided at references.
Having a properly lighted work area is essential to working safely. A couple of key points to remember about proper lighting:
- Lighting should be adequate for safe illumination of all work areas. TS EN 12464-1: 2013; TS EN 12464-1.2011: 2012 standards apply here.
- Light bulbs that are mounted low and susceptible to contact should be guarded.
- If the risk of electrocution exists when changing light bulbs, switch the connected power source prior to changing the bulb.
- For proper disposal of fluorescent bulbs (universal waste), please call LS or create an inquiry by calling 9988.
- As an energy conservation measure, please remember to turn off your lights when you leave your lab.
Temperature
The temperature conditions are established within thermal comfort of the users, in accordance with TS EN 27243 standard.
Noise
Exposure to excessive noise can permanently damage your hearing. The Noise at Work Regulations (TS 2607 ISO 1999 standard, Turkish Standards Institution, (2005). TS 2607 (ISO 1999). Acoustics-Determination of occupational noise exposure and estimation of noise-induced hearing impairment, Turkey.) Require that assessment of noise levels must be made by LSS wherever there is a noisy environment. In any cases of excessive noise, steps must be taken to reduce this at source. If this is not practicable, then ear protection must be made available to users, who must wear it in any prescribed areas of high noise level.
References and sources for information from the relevant websites and documentation of different universities, NGOs and government agencies used in the preparation of this website are provided at references.
All laboratories using hazardous chemicals , particularly corrosive chemicals, must have access to an eyewash and/or an emergency shower. Emergency eyewash and shower equipment must be readily accessible, free of obstructions and within immediate proximity to the hazard. The lid on the eyewash head must always be closed while not in use, to prevent dust particle accumulation
This important piece of emergency equipment is periodically inspected by LS, please report a detected malfunction to LS immediately.
For more information please see Emergency Procedures section.
Eyewash station and emergency shower are displayed on the figure below (Figure 4.6):

(Courtesy of Dekolab)
References and sources for information from the relevant websites and documentation of different universities, NGOs and government agencies used in the preparation of this website are provided at references.
Other Hazards
Detaylı bilgiGlassware is designed for a specific purpose. It ought to just be utilized for that reason. "Makeshift" device might be flimsy and could prompt mishaps and wounds.
While selecting glassware, decide the compatibility of the glass with the chemicals or procedure. Several chemicals react with glass or cause harm (etch) glass. On the off chance that your procedure includes temperature or pressure changes, make sure the glassware can withstand the progressions.
Volatile materials expand upon heating and result in explosion. An exothermic reaction takes place upon mixing sulfuric acid with water inside a barrel, bringing about the heat from the reaction to break the base of the vessel. Do not mix sulfuric acid inside a cylinder.
Hydrofluoric acid, hot phosphoric acid and strong hot alkalis attack and etch glassware. Do not use glass to carry out these processes.
Inspecting Glassware Before Use
Before working with glassware, dependably review it for imperfections. In the event that defects are found, glass ought to be expelled from administration. Scratches in glass can develop to cracks with time. Discard imperfect glassware if repairing is impractical.
Proper handling of glassware can reduce the risk of injury and accident.
- Never convey a flask by its neck. Never convey a beaker close by.
- Always carry glassware with two hands (support underneath with one hand).
- Appropriate gloves should be worn in case if there's a risk of breakage (e.g. embedding a glass bar), chemical contamination, or thermal hazard.
- When handling hot or cold glassware, always wear heat resistant gloves.
- Do not apply high pressure on glassware. Stabilize glassware by a clamp and platform to alleviate weight.
- Avoid over tightening and breakage while clamping the glassware. Use coated clamps to anticipate glass-to-metal contact.
- Neck clamps should not be utilized as primary support for vessels bigger than 500 mL.
- Ground-glass joints are made for a flawless fit and might stick infrequently.
- Never apply excess force on a joint free. It can bring the glass about to break.
- Lubricate surfaces using grease or use a Teflon sleeve.
- A heat gun can delicately release the joints.
- Never heat or cool glassware unless it is intended for those procedures.
- Round-bottom flasks are best for boiling fluids.
- Never set hot glass on a cold benchtop.
When storing glassware, remember to:
- Keep it away from shelf edges.
- Place glassware toward the back of benches or hoods. (Remember: Fume hoods and biosafety cabinets should not be used for storage.)
- Don't let instruments roll around in drawers (use drawer pads).
Setting up apparatus can involve pushing glass tubes through a cork or stopper. Users sometimes require a glass Pasteur pipette to rubber tubing attached for aspiration. These types of procedures possess high risk of injury to your hands if the glass tube, rod, or pipette breaks.
Some highlights to remember when working with glass rods, tubes or pipettes:
- Make sure that the glassware shafts of different connected glass materials fit with each other.
- Do not apply excessive force on a glass to put it into a place.
- Gently twist the glass material into place.
- Always lubricate the connections. You can use water, soapy water, or glycerin as lubricant. Using oil or grease is not advised.
- Wear cut resistant gloves when possible.
References and sources for information from the relevant websites and documentation of different universities, NGOs and government agencies used in the preparation of this website are provided at references.
Some glassware and processes can possess extra safety risks. Therefore, make sure you have had the relevant training prior to working with these kinds of specialized equipments or processes.
Vacuum or pressure operations can damage glassware severely. In such applications, walls of the container must be strong enough to withstand the changes in pressure. If the container is not able to withstand, the container may be broken. For those applications, a round bottomed or thick-walled flask must be employed. Glassware designed for vacuum or pressure operations are able to withstand certain pressure limits. Do not use glassware under pressure that it is more than it was designed to withstand.
Glassware that has been through repairs or has visible defects, flaw or damage should not be employed for vacuum systems applications. They are prone to break through thermal shock. It is important to check the glassware for flaws or defects prior to use.
Protective measures must be taken when setting up a vacuum system include:
- Put all vacuum apparatus inside a fume hood or behind a blast shield (Remember to use the fume hood in the lowest possible sash level).
- If possible, use PVC coated glassware. If not, cover flasks, dewars, and desiccators with a tape or mesh.
- Always wear appropriate personal protective equipment (safety goggles, face shield, and gloves).
Heating and Cooling Glassware
To determine safe temperature range of use, check with the manufacturer of glassware. Some glassware is intended to be used in to certain high and low temperatures. Using this glassware outside of their safe ranges may cause damage or breakage.
- Always keep an eye on evaporation process. A vessel may easily crack during evaporation.
- Never place hot glassware on cold or wet surfaces since it may be broken due to rapid temperature change.
- Never heat etched, cracked, nicked or chipped glassware.
- Never heat thick-walled glassware (e.g. bottles and jars) over a direct flame. Do not heat glassware directly on electrical heating elements.
- Do not try looking into a vessel being heated as evaporating material can damage your eye/skin.
- Unless mentioned otherwise, glassware should be cooled down slowly to prevent breakage.
- Pay extra attention while taking glassware out of low-temperature freezers (-70 to -150 ˚C) to prevent cracking and/or thermal shock. As a safe method, put the glassware under cold running water until thawing takes place. Do not transfer from the freezer directly into warm water baths.
- Remember that the flame of a Bunsen burner should touch the glass only below the liquid level. A ceramic wire gauze may help diffuse the flame therefore provide a more even heat.
- Hot plates used to heat the glassware should always be larger than the bottom of the vessel. Never heat thick-walled glassware (e.g. jars, bottles, cylinders, and filter flasks) on hot plates.
- Make sure that only the necessary setting is activated when using a hot/stir plates (i.e. if you do not intend to heat, make sure the hot plate is turned off.
References and sources for information from the relevant websites and documentation of different universities, NGOs and government agencies used in the preparation of this website are provided at references.
Good lab practices require the use of clean glassware. Glass must be both physically and chemically clean; even sterile for many cases. Most of the glassware accidents take place during cleaning. Following are some important reminders to wash and dry glassware:
- Personal protective equipments, especially eye protection goggles and chemically resistant gloves should be worn during washing.
- After using, glassware should be washed as quickly as possible. As the glassware left unwashed longer, it takes long time to clean. If necessary, use harder cleaning apparatus and keep and leave glassware in soapy water.
- Do not overload sinks, dishwashers, or soaking bins.
- Keep glassware clear of the sides of the sink.
- Do not use worn out brushes as they may scratch the glass.
- In case if you need to use caustic for cleaning purposes, it is advised to ask help from an experienced lab user in the safe usage of caustic cleaning agents, especially prior to using aqua-regia, chromic acid or other reactive solutions.
- Leave glassware drying on towels, lined basket, or slip-resistant pads. Place glassware away from the edge of the bench. Large containers may be hung on pegs to dry.
- Pipettes and tips may be cleaned in a cylinder or tall jar of water with appropriate disinfectant (e.g. for biologically contaminated tips). To prevent tips from breakage, you may put a cotton pad or glass wool at the bottom.
- To remove any residue or loose particles, new glassware should be washed prior to use.
References and sources for information from the relevant websites and documentation of different universities, NGOs and government agencies used in the preparation of this website are provided at references.
Spills and Broken Glass
Glass is fragile and breaks easily. Care should be taken to reduce the risks of health when a glass breaks.
- If something is falling, let it drop! Trying to catch it may result in glassware to be broken in your hand.
- While handling broken glass, wear cut-resistant gloves whenever possible. Disposal nitrile or latex gloves should never be worn. Glass will cut through those gloves.
- When cleaning broken glass, use mechanical means to pick up the pieces.
- Do not pick up broken glass with bare hand. Use tongs, tweezers, or forceps to collect broken glass pieces.
Disposal
Proper disposal of broken glass ensures safety of others. In case of dealing with contaminated broken glass:
- Collect broken glass in a rigid, puncture-resistant container (e.g. sharps container).
- Place biologically contaminated broken glass in a closed and sealed container and put in biohazardous waste box for disposal.
- Place chemically contaminated broken glass in a closed and sealed containers.
- Dispose uncontaminated broken glass of in a broken glass box or uncontaminated waste box.
For more information please see Waste Management section.
References
- University of Vermont, Safety in Laboratories, Identifying the Hazards: Safe Handling of Glassware. (2015, March). Retrieved from http://www.uvm.edu/safety/lab/safe-handling-of-glassware
References and sources for information from the relevant websites and documentation of different universities, NGOs and government agencies used in the preparation of this website are provided at references.
Safety Data Sheet
Detaylı bilgiGeneral Chemical Procedures
Detaylı bilgiChemical Exposure Monitoring
Detaylı bilgiChemical Spills
Detaylı bilgiCompressed Gas Safety
Detaylı bilgiCarcinogenic, Reproductive And Highly Toxic Chemicals
Detaylı bilgiChemical Storage Guidelines
Detaylı bilgiGeneral Specifications
Detaylı BilgiCare And Use Of Electrical Systems
Detaylı bilgiPreventing Electrical Hazards
Detaylı bilgiGeneral Safety Rules
Detaylı bilgiSafety Issues When Using Machine Tools
Detaylı bilgiSafety Issues When Using Hand And Power Tools
Detaylı bilgiStrains, sprains, fractures and bruises are common injuries related to the handling of materials. They are caused primarily by improper lifting techniques, carrying too heavy of a load, incorrect grip, failure to observe foot and hand clearances, and failure to wear protective equipment.
- Inspect material for slivers, burrs, and rough or sharp edges.
- Keep your hands free of oil or grease, wipe off any grease or slippery objects before you move them.
- Keep a firm grip on the object to be moved.
- If an object is heavy or clumsy get help lifting it. Discuss beforehand how the object is to be moved and set down.
- Be sure the path over which an object is to be moved is clear of obstructions before you start.
- Always lift with your legs and not your back.
- Watch pinch points, especially when setting the object down.
This guide presents a summary of the basics of laser safety, biological effects, and exposure limits to be used at Sabancı University. This guide applies to all laser classes (1 to 4). Some recommendations will improve laser safety in the laboratory. Because of the wide variety of lasers and laser uses that are possible, this guide provides performance based goals rather than prescriptive requirements.
Each laser user is responsible for the operational safety of the device. This includes applicable recommendations of the position and termination of all beams and reflections, using appropriate entryway controls, and using appropriate eye protection. Laser users shall not bypass or defeat entryway safety features, barriers, or interlocks. Doing so is a serious safety violation and may lead to loss of laser use privileges.
The international and national resources for laser applications and safety are given below:
- TS EN 60825-4/A1 - Safety of laser products - Part 4: Laser guards
- TS-EN-60825-1 - Safety of laser products - Part 1: Equipment classification and requirements (IEC 60825-1:2014)
- ANSI Z136 Standards (Laser Institute of America)
Training
Detaylı BilgiLaser Classes And Hazards
Detaylı BilgiWorking With Lasers
Detaylı BilgiLaser Clearance And Blackout Curtains
Detaylı BilgiEmergency Procedures
Note that there are no visible evidences of oxygen-deficient atmospheres. They don’t have warning properties. Always carry an air source while entering a suspected oxygen-deficient area. Carry air-monitoring devices to measure oxygen levels on site. When it is necessary to work in an oxygen-deficient area, supplied air must be provided. Should a Dewar of cryogenic liquid be venting continuously, call LS/LSS immediately.
For further information please click