Why is the 2022 Nobel Prize in Chemistry important for drug research?
Nur Mustafaoglu
This year's Nobel Prize in Chemistry was shared by three scientists: Carolyn Bertozzi for ortogonal chemistry, Barry Sharpless and Morten Meldal for click chemistry. Sharpless developed a brand-new field of chemistry. Meldal discovered a reaction that illustrates how the combination of certain chemicals can trigger a click reaction. Bertozzi created a new class of biological drugs to fight cancer cells. The work of these three scientists was groundbreaking because the new method of chemistry enabled the efficient production of novel compounds, which is critical for material science and drug discovery.
The American scientist Sharpless is the fifth person to win two Nobel Prizes; he is the only living person with two Nobel prizes. He was known for his pioneering work in the field of asymmetric synthesis, i.e., the development of strategies for the effective and selective synthesis of complex compounds. Sharpless received the Nobel Prize in Chemistry in 2001 for his efforts in this field. Sharpless has made numerous important contributions to chemistry, and his work has significantly influenced the development of new drugs and other complicated compounds. This year he received he prize for click chemistry [1].
What is click chemistry?
Click chemistry is a term used to describe a series of chemical reactions that are fast, efficient, and highly selective. This new type of chemistry usually involves the use of small, stable molecules that can be easily and selectively combined to form larger, more complex structures. These reactions are often carried out under mild conditions, making them suitable for a wide range of applications. Click chemistry has revolutionized the way chemists synthesize complex molecules and has numerous potential applications in fields such as medicine, materials science and biotechnology. These reactions are commonly used in the synthesis of complex molecules, such as drugs or other biologically active compounds.
The "crown jewel" of click chemistry was discovered separately by Meldal and Sharpless and revolutionised chemistry. Copper-catalyzed azide-alkyne cycloaddition is the name of this reaction. An alkyne is an unsaturated hydrocarbon with a triple bond, while an azide is a chemical containing the N3 molecule. In a very typical reaction, Meldel, searching for possible medicinal compounds, attempted to react an alkyne with an acyl halide, an organic molecule containing a halogen atom. To achieve a smooth reaction, scientists must add palladium and copper ions as catalysts. 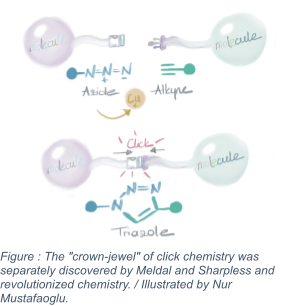 Meldal and his collaborators, however, noticed a surprising result. The wrong end of the acyl halide was where the reaction of the alkyne took place. At that end was an azide. The triazole structure was prepared by combining alkyne and azide molecules. Triazoles are stable and practical chemical structures found in various colors, medicines, and agricultural preparations. Although scientists had attempted to make triazoles before, the processes also produced undesirable byproducts. The reaction was regulated because Meldal had used copper ions, resulting in the formation of only one chemical. As a result, the acyl halide remained unchanged in the reaction vessel. It was the "perfect" click reaction [2-3]. The reaction is consistent and works in water.
Meldal and his collaborators, however, noticed a surprising result. The wrong end of the acyl halide was where the reaction of the alkyne took place. At that end was an azide. The triazole structure was prepared by combining alkyne and azide molecules. Triazoles are stable and practical chemical structures found in various colors, medicines, and agricultural preparations. Although scientists had attempted to make triazoles before, the processes also produced undesirable byproducts. The reaction was regulated because Meldal had used copper ions, resulting in the formation of only one chemical. As a result, the acyl halide remained unchanged in the reaction vessel. It was the "perfect" click reaction [2-3]. The reaction is consistent and works in water.
Only in the presence of copper ions could an azide be bound to an alkyne in the azide-alkyne cycloaddition catalyzed by copper. The reaction cannot take place within living organisms because copper is harmful to them. Bertozzi discovered that studies in 1961 had shown that azides and alkynes can react virtually explosively without the aid of copper. This is possible when the alkyne is pressed into a chemical structure that resembles a ring, creating a tension that generates enough energy to ensure a smooth reaction [4]. That was the birth of copper-free click reaction, biorthogonal chemistry [5].
What is orthogonal chemistry?
Ortogonal chemistry is often used in combination with other techniques such as click chemistry to create a wide range of complex structures. Ortogonal chemistry is a term used to describe a type of chemical reaction that is highly selective, efficient, and predictable. Ortogonal reactions are usually carried out under mild conditions and involve the use of small, stable molecules that can be easily combined to form larger, more complex structures. Because these reactions can be carried out selectively under mild conditions, they can also take place within a living system without interacting with or disrupting the natural biochemistry of the system. These interactions, which take place without disturbing the normal chemistry of the cell, have profound effects on biochemistry. These reactions are often used in the synthesis of complex molecules, such as drugs or other biologically active compounds.
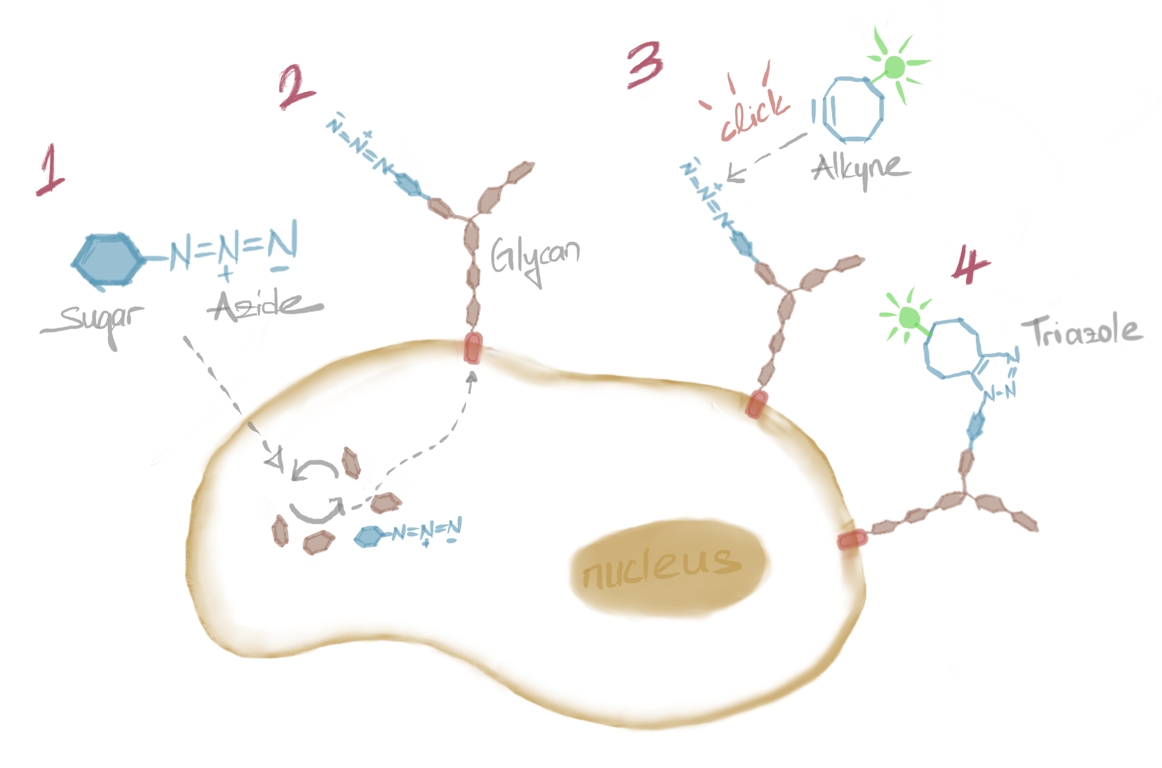
Caroline Bertozzi is known for her pioneering work in the field of glycobiology, the study of carbohydrates and their role in biological processes. She has contributed significantly to our understanding of the structure and function of glycans. Glycans are complex sugars that play an important role in many biological processes. Caroline Bertozzi's work in the field of glycobiology has been revolutionary, providing new insights into the structure and function of glycans. Bertozzi's research has helped uncover the fundamental role of glycans in a variety of biological processes, including cell signaling, immune response and development. This work has led to a better understanding of the role of glycans in health and disease and laid the foundation for the development of new treatments for a variety of diseases. Bioorthogonal chemistry was the heart of her research.
Both click and bioorthogonal reactions are incredibly useful to civilization because they can be used to create new materials. For example, to develop materials that conduct electricity, have antibacterial properties, absorb sunlight and block UV rays, some manufacturers add a clickable azide to a fiber or plastic. Bertozzi's work is an excellent example of the application of click chemistry in the pharmaceutical field [6-7]. While researching glycans, she discovered that glycans appear to protect tumors from the immune system by shutting down immune cells. To turn off this protective mechanism, Bertozzi and her collaborators developed a new class of biologic drugs.
In recent works, some scientists are working to develop clickable antibodies that attach to specific tumors and then attack them [8]. The next step is to inject a second molecule that binds to the antibody. Using positron emission tomography (PET), this chemical could be a radioisotope that can monitor tumor growth. Another possibility is to use a chemical that directs a lethal dose of radiation at the cancer cells. In this way, humanity benefits greatly from click reactions and bioorthogonal chemistry.
References:
[1] Kolb, Hartmuth C., M. G. Finn, and K. Barry Sharpless. "Click chemistry: diverse chemical function from a few good reactions." Angewandte Chemie International Edition 40.11 (2001): 2004-2021.
[2] Tornøe, Christian W., Caspar Christensen, and Morten Meldal. "Peptidotriazoles on solid phase:[1, 2, 3]-triazoles by regiospecific copper (I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides." The Journal of organic chemistry 67.9 (2002): 3057-3064.
[3] Meldal, Morten, and Christian Wenzel Tornøe. "Cu-catalyzed azide− alkyne cycloaddition." Chemical reviews 108.8 (2008): 2952-3015.
[4] Agard, Nicholas J., Jennifer A. Prescher, and Carolyn R. Bertozzi. "A strain-promoted [3+ 2] azide− alkyne cycloaddition for covalent modification of biomolecules in living systems." Journal of the American Chemical Society 126.46 (2004): 15046-15047.
[5] Sletten, Ellen M., and Carolyn R. Bertozzi. "Bioorthogonal chemistry: fishing for selectivity in a sea of functionality." Angewandte Chemie International Edition 48.38 (2009): 6974-6998.
[6] Kolb, Hartmuth C., and K. Barry Sharpless. "The growing impact of click chemistry on drug discovery." Drug discovery today 8.24 (2003): 1128-1137.
[7] Bertozzi, Carolyn. "A Special Virtual Issue Celebrating the 2022 Nobel Prize in Chemistry for the Development of Click Chemistry and Bioorthogonal Chemistry." ACS Central Science (2022).
[8] Chio, Tak Ian, and Susan L. Bane. "Click Chemistry Conjugations." Antibody-Drug Conjugates. Humana, New York, NY, 2020. 83-97.